Malaria in Pregnancy: An Unrecognized Risk Factor for Neuropsychiatric Disease in Offspring?
Vanessa Tran, Andrea Weckman and Kevin C. Kain*
DOI10.21767/2573-0282.100016
1Sandra A. Rotman Laboratories, Sandra Rotman Centre for Global Health, Toronto General Research Institute-University Health Network,
Toronto, Ontario, Canada
2Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada
3Department of Medicine, Tropical Disease Unit, Division of Infectious Diseases, University of Toronto, Toronto, Ontario, Canada
- *Corresponding Author:
- Kevin C. Kain
Department of Medicine
Tropical Disease Unit
Division of Infectious Diseases
University of Toronto,
Toronto, Ontario
Canada
Email: kevin.kain@uhn.ca
Received date: May 28, 2016; Accepted date: June 10, 2016; Published date: June 13, 2016
Citation: Kain KC, Weckman A and Tran V (2016) Malaria in Pregnancy: An Unrecognized Risk Factor for Neuropsychiatric Disease in Offspring? Pediatric Infect Dis 1:16. doi: 10.21767/2573-0282.100016
Abstract
The in vitro environment has a profound effect on offspring neurodevelopment. Insults to the foetus during pregnancy can disrupt neurodevelopmental processes culminating in neurochemical and behavioural abnormalities. A growing body of evidence supports a role for maternal infection in precipitating neuropsychiatric disease in offspring. Since ~60% of pregnancies globally are at risk of malaria infection annually, we hypothesize that in utero exposure to malaria in pregnancy (MIP) may be a neglected risk factor for mental illness. We propose that the host response to infection makes an important contribution to this risk. MIP may mediate neuropathology via immune activation and inflammation in the placenta and consequent dysregulation of processes critical to fetal neurodevelopment including cerebral angiogenesis, neurogenesis, synaptic pruning, and neurochemical regulation.
Keywords
Malaria, Pregnancy, Maternal infection, Neuropsychiatric disease
Abbreviations
ASD: Autism Spectrum Disorder; BD: Bipolar Disorder; DALY: Disability-Adjusted Life Years; FGR: Fetal Growth Restriction; LBW: Low Birth Weight; VLBW: Very Low Birth Weight; MIP: Malaria In Pregnancy; PM: Placental Malaria; Poly(I:C): Polyriboinosinic-Polyribocytidilic acid; PTB: Preterm Birth; SGA: Small For Gestational Age
The Global Burden of Mental Illness
Neuropsychiatric disease is an enormous contributor to the global financial and social burden of health. According to a 2015 meta-analysis, an estimated 8 million deaths, representing 14.3% of global mortality, can be attributed to neuropsychiatric disease [1]. Even more importantly, neuropsychiatric disease is the leading cause of disabilityadjusted life years (DALYs) worldwide, with 96 million DALYs attributed to schizophrenia, depression, and bipolar disorder (BD) alone [2]. The financial cost of mental illness in low- and middle-income countries in 2010 was estimated at $870 billion USD and this number is expected to double by 2030 [2]. The majority of this cost is allocated to treatment. To reduce this staggering burden, resources and interventions that focus on prevention of neuropsychiatric disease would likely have more impact and reduce the long-term costs. Mounting pre-clinical and epidemiological evidence has implicated maternal infection as a risk factor for developing neuropsychiatric diseases including autism spectrum disorder (ASD), schizophrenia, BD, and depression [3].
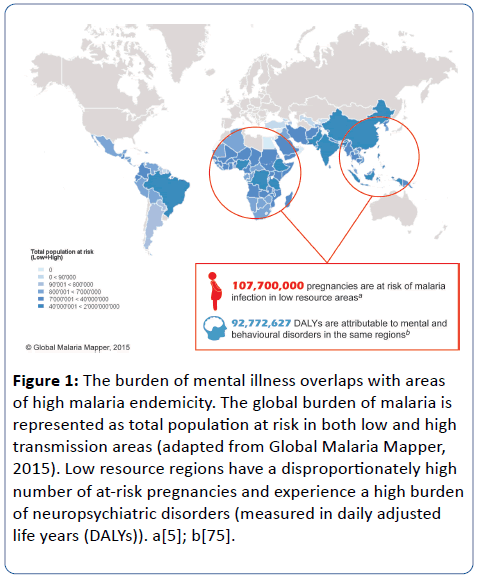
Notably, low and middle income countries, including most sub-Saharan African countries, have a disproportionately high burden of mental illness, which has often been attributed to a number of social determinants and a lack of effective treatments and health care professionals, such as psychiatrists [4]. While these social factors likely contribute, maternal infection may represent an important and underappreciated risk factor. Given the high prevalence of malaria in these regions, we propose that the disproportionate burden of mental illness in low and middle income countries may also be associated with MIP and hypothesize that prenatal malaria exposure is a modifiable risk factor for neuropsychiatric disorders later in life (Figure 1). MIP has an established role in adverse birth outcomes including low birth weight (LBW) and stillbirth. Re-thinking MIP as a preventable risk factor for childhood neurocognitive and adult neuropsychiatric disorders has profound global health implications and could galvanize efforts to prevent maternal infections. Here, we review the evidence for the maternal infection hypothesis of psychiatric disease and examine the potential for MIP to contribute to the global burden of neuropsychiatric disorders.
Figure 1: The burden of mental illness overlaps with areas of high malaria endemicity. The global burden of malaria is represented as total population at risk in both low and high transmission areas (adapted from Global Malaria Mapper, 2015). Low resource regions have a disproportionately high number of at-risk pregnancies and experience a high burden of neuropsychiatric disorders (measured in daily adjusted life years (DALYs)). a[5]; b[75].
Adverse Birth Outcomes in Placental Malaria
Approximately 125 million pregnant women are at risk of MIP each year and over 85 million of these occur in areas with stable Plasmodium falciparum transmission [5]. MIP can result in placental malaria (PM), which is characterized by the accumulation of malaria-infected erythrocytes and monocytes in the placenta. PM can have negative health consequences for mother and child, including maternal anaemia, stillbirth, and LBW caused by preterm birth (PTB) and/or fetal growth restriction (FGR) [6]. PM-mediated morbidity and mortality is influenced by parity, maternal immune status, stage of pregnancy at infection, and chronicity of infection [7,8].
These adverse birth outcomes are thought to result, at least in part, from increased inflammation at the maternal-fetal interface. Sequestration of parasitized erythrocytes in the intervillous space of the placenta triggers the secretion of chemotactic β-chemokines by mononuclear cells, resulting in an accumulation of monocytes and macrophages, and a deviation from the normal cytokine profile in the placenta [9,10]. For example, pro-inflammatory cytokines such as TNF are increased in PM and have been associated with poor birth outcomes [9,11].
A central initiator and enhancer of the localized inflammatory response in PM is complement. The complement system is an essential component of innate immunity and the host defence against pathogens. However, dysregulated activation or control of the complement system is associated with several adverse pregnancy outcomes, such as preeclampsia and spontaneous abortion [12,13]. Human, pre-clinical models, and in vitro studies have shown that PM is associated with increased activation of the complement system, in particular enhanced generation of the proinflammatory component C5a [14,15]. In vitro, C5a can induce the synergistic release of both inflammatory mediators (e.g. TNF, IL-6, IL-8, MCP-1) and anti-angiogenic factors (e.g. sFlt-1) from human mononuclear cells [15]. Moreover in pre-clinical and clinical studies increased levels of C5a were associated with dysregulation of angiogenic factors (e.g. VEGF, endoglin, angiopoietin-1 and angiopoietin-2), placental vascular insufficiency, and an increased risk of adverse birth outcomes including LBW and stillbirth [14,15].
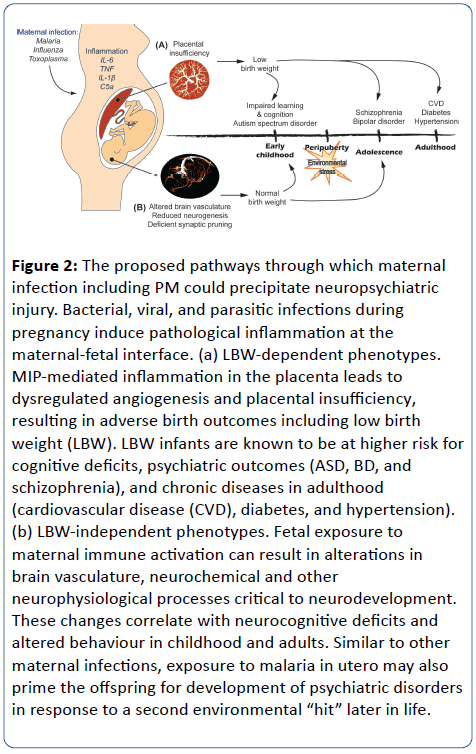
PM-mediated LBW has a marked impact on neonatal mortality and is estimated to contribute to 200,000 infant deaths per year [16]. Even for those babies that survive the neonatal period, however, numerous epidemiological and animal studies have linked LBW with long-term neurodevelopmental deficits and adult disease (Figure 2).
Figure 2: The proposed pathways through which maternal infection including PM could precipitate neuropsychiatric injury. Bacterial, viral, and parasitic infections during pregnancy induce pathological inflammation at the maternal-fetal interface. (a) LBW-dependent phenotypes. MIP-mediated inflammation in the placenta leads to dysregulated angiogenesis and placental insufficiency, resulting in adverse birth outcomes including low birth weight (LBW). LBW infants are known to be at higher risk for cognitive deficits, psychiatric outcomes (ASD, BD, and schizophrenia), and chronic diseases in adulthood (cardiovascular disease (CVD), diabetes, and hypertension). (b) LBW-independent phenotypes. Fetal exposure to maternal immune activation can result in alterations in brain vasculature, neurochemical and other neurophysiological processes critical to neurodevelopment. These changes correlate with neurocognitive deficits and altered behaviour in childhood and adults. Similar to other maternal infections, exposure to malaria in utero may also prime the offspring for development of psychiatric disorders in response to a second environmental “hit” later in life.
The Impact of Low Birth Weight on Neurodevelopment
Over 20 million babies are born LBW each year, the majority of which are in low resource countries [17]. LBW can occur from either PTB and/or being small for gestational age (SGA). PTB is the leading cause of childhood mortality worldwide, accounting for over one million deaths [18,19]. Further, LBW from PTB is associated with greater mortality and more severe outcomes, such as cerebral palsy, than LBW caused by SGA. The etiology of PTB and SGA varies by region and is multifactorial including tobacco and drug use during pregnancy, malnutrition, maternal infection, hypertension, and preeclampsia [20,21]. Both PTB and SGA have been linked to significant neonatal morbidity and long-term adverse health outcomes.
Numerous epidemiological studies have identified an association between restricted fetal growth with poor growth in childhood and a higher incidence of chronic diseases in adulthood. This correlation between LBW and adult disease is known as the “Barker Hypothesis” of developmental plasticity and programming [22,23]. Adult chronic diseases associated with this phenomenon include obesity, coronary artery disease, hypertension, and type 2 diabetes [22]. LBW has also been associated with impaired neurodevelopment leading to cognitive and behavioural deficits in childhood and adolescence. For example, a meta-analysis of case-control studies of PTB children found that decreasing birth weight and decreasing mean gestational age at birth correlated with lower cognitive test scores. Further, children born preterm and very LBW (VLBW) (< 1500g) were twice as likely to develop attention-deficit/hyperactivity disorder (ADHD) and exhibit autism-like symptoms [24,25]. In another study, very preterm (≤ 33 weeks gestation) and VLBW infants had significant deficits in academic achievement, including mathematics and reading performance, and poor executive function compared to their full term, normal birth weight peers [26]. These learning and behavioural impairments impact school performance and can persist to adulthood.
In addition to deficits in cognition and learning, epidemiological studies have associated LBW with an increased risk for neuropsychiatric and affective disorders later in life. For example, a Norwegian study examined psychiatric outcomes in adolescents born preterm and VLBW and reported that LBW was associated with reduced psychosocial function and an increase in ADHD symptoms [27]. Similar to other epidemiological studies, these findings were significant after adjusting for confounding factors, including maternal mental illness, gender, and social economic status. Further, population-based studies indicate that these deficits can persist into adulthood. The Helsinki Study of Very Low Birth Weight Adults found that adults who were born preterm and VLBW had an increased risk of ASD, represented by a higher social interaction sum score and a lower attention to detail score [28]. Findings from these epidemiological studies have been supported by animal models, where rodent models of FGR using maternal food restriction showed heightened anxiety-like behaviour in offspring that were LBW [29,30].
It is clear that PM-mediated LBW has both short- and longterm negative health consequences. However, most malaria in pregnancy result in normal birth weight infants, but whether these babies have neurocognitive and behavioural deficits is unknown. However accumulating evidence supports the hypothesis that even pauci-symptomatic maternal infections may represent an environmental “hit” that may precipitate or predispose offspring to neuropsychiatric disorders later in life [3].
Maternal Infection and Impact on Neurodevelopment
Numerous factors contribute to the development of neuropsychiatric disorders, including genetic risk alleles, epigenetic changes, and environmental triggers. In addition to these, a growing body of epidemiological and pre-clinical evidence supports a link between prenatal exposure to maternal viral and bacterial infections, and several neuropsychiatric disorders including schizophrenia, ASD, and BD [3,31–34]. The first association between maternal infection and an increased risk for psychotic disorders came from the observation of a seasonal pattern in birth dates among patients with schizophrenia and BD [35–37]. Consistently, epidemiological studies found that adults born during an influenza epidemic had an increased risk of schizophrenia [35,38]. More rigorous prospective studies using serologic detection of prenatal influenza infection further supported this link [39]. Clinical evidence of fetal exposure to maternal infections with Toxoplasma gondii and herpes simplex virus (type II) and an increased risk of adult schizophrenia have also been found [40–42]. Evidence from large birth cohorts have emerged that further support an association between maternal infection and an increased risk for other neuropsychiatric disorders including BD, ASD, and posttraumatic stress disorder [3,32,43–45].
Ethical and logistical considerations limit the ability to examine causality in human epidemiological studies. Therefore, the use of animal models has enabled mechanistic and causal studies of maternal infection inducing neuropsychiatric disorders. These animal studies have supported and extended the findings from human populationbased epidemiological studies. These models have exploited the use of whole virus (influenza), the viral mimic polyriboinosinic-polyribocytidilic acid (poly (I:C)), and bacterial endotoxin lipopolysaccharide (LPS), to trigger systemic maternal immune activation [33,46]. In addition to these models of systemic maternal infection in pregnancy, turpentine intramuscular administration has been used to model local maternal inflammation, which has enabled the exploration of maternal versus fetal contributions to neurobehavioral outcomes [47]. Importantly, animal models permit control of confounding factors and have enabled the study of single infectious agents and/or inflammatory factors as well as the contribution of genetic risk and environmental factors in precipitating neuropsychiatric outcomes.
Mechanisms of Impaired Neurodevelopment Following in Utero Exposure to Infection
Evidence from human and animal studies support the hypothesis that maternal immune activation in response to infection underlies the persistent immunological and neurological alterations in the offspring that contribute to neuropsychiatric outcomes. Neurodevelopment is a complex and tightly regulated process that requires multiple orchestrated steps to achieve functional neural networks and the refinement of the central nervous system (CNS) required for specific connectivity and neurotransmission. Multiple lines of evidence support a critical non-immune role for the complement system and cytokines in both normal and abnormal neurodevelopmental processes (i.e. synaptic pruning, synaptic refinement, regulation of neural stem cell pools, neurodegenerative diseases) [13,48,49]. Maternal inflammation at the placental-fetal interface can induce an inflammatory response in the foetus. Several cytokines, including IL-6, are able to cross the placenta and fetal bloodbrain barrier [50], placing the developing foetus at risk of an imbalance of critical neurodevelopmental factors. IL-6 is frequently induced during prenatal infections and has been detected at elevated levels in maternal serum, placenta, and fetal brain in the poly (I:C) model of maternal infection [51]. Mechanistically, IL-6 is critically involved in the regulation of neural stem cell pools and neurogenesis [48], and elevated levels can contribute to aberrations in these developmental processes, resulting in long term neurological deficits [51]. This contention is supported by the findings that functional blockade (e.g. anti-IL-6 antibody) or genetic disruption of IL-6, in poly (I:C)-treated dams prevented behavioural deficits in the exposed offspring [51]. Other pro-inflammatory cytokines or factors that have been implicated in abnormal neurodevelopment include TNF, IL-8/CXCL8, IL-1β, and CRP [52–54]. The use of single cytokine-induced systemic inflammation has helped to define their specific roles in the maternal infection model of neuropsychiatric disease. For example, a single IL-6 injection in pregnant mice elicited behavioural phenotypes in the offspring similar to the poly(I:C) or LPS models [51]. Inflammation at the fetal-maternal interface has also been reported to damage the blood-brain barrier leading to pathological neuro-inflammation, neuronal cell death, and white matter injury [55]. Other immunological mediators have also been implicated in the aberrant neurodevelopment resulting from maternal immune activation. Specifically, synaptic pruning and refinement, processes that are crucial for normal brain function, are known to require the complement protein C1q and MHC class 1 [56–58]. Altered levels of these proteins have been associated with abnormal neuronal connectivity, disturbances in neurotransmitter systems, and abnormal behaviour. Maternal infection is also associated with hypomyelination in offspring, leading to abnormal brain white matter, as well as impaired neuronal migration due to reduced reelin expression, a key regulator of neuronal migration in the CNS [59,60]. A recent study reported that maternal malaria infection was associated with decreased levels of brain-derived neurotropic factor (BDNF) a critical regulator of neuron growth and remodelling, synapse formation, and neurotransmitter release [61,62]. Decreased BDNF was associated with a subsequent decrease in regional neurotransmitter levels that correlated with neurocognitive and behavioural deficits in offspring [63].
Early perturbations to CNS development would be expected to promote long-term alterations in neurophysiology and subsequent behaviour. Consistent with this model, patients with ASD and schizophrenia often exhibit abnormal immunology, including increased levels of IL-6 and TNF in circulation and in cerebrospinal fluid, in adulthood [64,65]. This phenomenon is hypothesized to occur through microglia priming. Microglia is CNS resident macrophages that act as primary immune mediators. Maternal immune activation with poly (I:C) or LPS results in persistent microglia alterations in the offspring that can be hyper-reactive to subsequent stimuli [53,66]. This observation aligns with the “two-hit” hypothesis of neuropsychiatric disease, which posits that early neurodevelopmental interference such as maternal infection primes the offspring for neuropsychiatric disorders in response to a “second hit" (i.e. stress, infection, trauma, drugs) later in life [67]. Accordingly in pre-clinical models a secondary insult, such as peripubertal stress or postnatal poly (I:C) challenge, following prenatal exposure to an immune stimulus resulted in hyperactive microglia, intensified inflammatory response, and schizophrenia-like behaviour [68,69].
Malaria in Pregnancy and Neurocognitive Outcomes
Given the mounting evidence to support the link between in vitro exposure to maternal infections and an increased risk for neuropsychiatric disorders, we hypothesize that MIP can also prime the offspring for detrimental neurological outcomes. Recent evidence indicates that mice exposed to malaria in vitro exhibit neurological injury and importantly, this occurs in the absence of LBW or other birth phenotype. Malariaexposed, pre-pubescent mice displayed increased depressivelike behaviour and deficits in learning and memory [63]. These behavioural and cognitive deficits correlated with abnormal fetal brain vasculature, decreased BDNF expression and reduced regional neurotransmitter levels of dopamine, serotonin and norepinephrine. CNS angiogenesis and neurogenesis are co-regulated and abnormal neurovasculature development could contribute to altered neurogenesis and lasting neurological injury in offspring. Importantly, the PMexposed mice were not congenitally infected, consistent with the idea that the host response to the pathogen, rather than the pathogen itself, plays a major role in driving the observed adverse fetal outcomes. Further, disruption of C5a-C5aR signalling rescued neurocognitive deficits and restored neurochemical balance in the exposed offspring [63]. Given the central role of C5a in inflammation, and the importance of brain vascularization in normal neurodevelopment, it is reasonable to hypothesize that these immune mechanisms of injury could also contribute to neuropsychiatric disorders.
The neurochemical changes in malaria-exposed offspring are consistent with reports of altered brain chemistry in neuropsychiatric disorders [62,70,71]. In further support of this hypothesis, several inflammatory factors including IL-6, TNF and CRP that are implicated as mediators of maternal immune activation-related neuropsychiatric disorders [51,53,54,72] are increased in MIP [9,15,73,74].
Concluding Remarks
Maternal infection and its impact on the in vitro environment, represents a paradigm shift in our understanding of the risk factors and putative mechanisms underlying neurocognitive and neuropsychiatric disorders and suggest that MIP may be a potential and preventable risk factor. Collectively the emerging evidence defining links between maternal infection and neuropsychiatric disorders in offspring has important implications for the cause and prevention of what were previously believed to be non-communicable diseases, and may shift global health priorities from costly lifelong treatment and rehabilitation to prevention. It has been estimated that prevention of viral, bacterial, and parasitic infections during pregnancy could prevent more than 30% of schizophrenia cases [35]. Factors that contribute to neurological injury in infants place enormous social and financial burdens on families and communities, especially in low-resource settings. In the case of MIP, neurological injury can occur via both LBW and LBW-independent pathways (Figure 2). Similar to Zika infection in pregnancy, the nature of this injury may depend on the timing of infection since the stages of fetal neurodevelopment correlate with different stages of gestation [3]. For example, malaria infection during the first trimester could more profoundly impact neurogenesis, whereas infection later in gestation may disrupt neuronal migration or myelination. Whether this timing might cause differing neuropsychiatric outcomes is unknown [3]. Clearly additional prospective and mechanistic studies are required to determine and define the potential risk of MIP in human populations. However, given the high prevalence of malaria in many low-resource settings, ultimately, targeting MIP may represent a cost-effective strategy for the concurrent reduction of maternal and childhood morbidity and mortality, as well as juvenile and adult mental illness.
Funding
This review was supported in part by the Canadian Institutes of Health Research [CIHR MOP- 136813 and MOP-115160 to KCK]; Canadian Research Chair (KCK); Global Alliance to Prevent Prematurity and Stillbirth and the Bill and Melinda Gates Foundation, Grand Challenges in Global Health: Preventing Preterm Birth Initiative Grant No. 12003 [KCK]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Walker ER, McGee RE, Druss BG (2015) Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry 72: 334-341.
- Bloom DE, Cafiero E, Jané-Llopis E, Abrahams-Gessel S, Bloom LR, et al. (2011) The Global Economic Burden of Noncommunicable Diseases. Geneva World Econ Forum; Available: https://ideas.repec.org/p/gdm/wpaper/8712.html
- Knuesel I, Chicha L, Britschgi M, Schobel SA, Bodmer M, et al. (2014) Maternal immune activation and abnormal brain development across CNS disorders. Nat Rev Neurol 10: 643-660.
- Collins PY, Patel V, Joestl SS, March D, Insel TR, et al. (2011) Grand challenges in global mental health. Nature 475: 27-30.
- Dellicour S, Tatem AJ, Guerra CA, Snow RW, TerKuile FO (2010) Quantifying the number of pregnancies at risk of malaria in 2007: a demographic study. PLoS Med 7: e1000221.
- Desai M, terKuile FO, Nosten F, McGready R, Asamoa K, et al. (2007) Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis 7: 93-104.
- Kalilani L, Mofolo I, Chaponda M, Rogerson SJ, Meshnick SR (2010) The effect of timing and frequency of Plasmodium falciparum infection during pregnancy on the risk of low birth weight and maternal anemia. Trans R Soc Trop Med Hyg 104: 416-422.
- Stanisic DI, Moore KA, Baiwog F, Ura A, Clapham C, et al. (2015) Risk factors for malaria and adverse birth outcomes in a prospective cohort of pregnant women resident in a high malaria transmission area of Papua New Guinea. Trans R Soc Trop Med Hyg 109: 313-324.
- Umbers AJ1, Aitken EH, Rogerson SJ (2011) Malaria in pregnancy: small babies, big problem. Trends Parasitol 27: 168-175.
- Rogerson SJ, Hviid L, Duffy PE, Leke RF, Taylor DW (2007) Malaria in pregnancy: pathogenesis and immunity. Lancet Infect Dis 7: 105-117.
- Suguitan AL, Leke RG, Fouda G, Zhou A, Thuita L, et al. (2003) Changes in the levels of chemokines and cytokines in the placentas of women with Plasmodium falciparum malaria. J Infect Dis 188: 1074-1082
- Lynch AM, Salmon JE (2010) Dysregulated complement activation as a common pathway of injury in preeclampsia and other pregnancy complications. Placenta 31: 561-567.
- McDonald CR, Elphinstone RE, Kain KC (2013) The impact of placental malaria on neurodevelopment of exposed infants: a role for the complement system? Trends Parasitol 29: 213-219.
- Conroy AL, Silver KL, Zhong K, Rennie M, Ward P, et al. (2013) Complement activation and the resulting placental vascular insufficiency drives fetal growth restriction associated with placental malaria. Cell Host Microbe 13: 215-226.
- Conroy A, Serghides L, Finney C, Owino SO, Kumar S, et al. (2009) C5a enhances dysregulated inflammatory and angiogenic responses to malaria in vitro: potential implications for placental malaria. PLoS One 4: e4953.
- Steketee RW, Nahlen BL, Parise ME, Menendez C (2001) The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg 64: 28-35.
- https://www.who.int/nutrition/publications/globaltargets2025_policybrief_lbw/en/
- March of Dimes, The Partnership for Maternal Newborn& Child Health, Save the Children, WHO (2012) Born Too Soon: The Global Action Report on Preterm Birth. In: Howson C, Kinney M, Lawn J, editors. Geneva: World Health Organization.
- https://www.who.int/gho/publications/world_health_statistics/2015/en/
- Kramer MS (2013) The epidemiology of low birthweight. Nestle NutrInst Workshop Ser 74: 1-10.
- https://www.unicef.org/publications/index_24840.html
- Calkins K, Devaskar SU (2011) Fetal origins of adult disease. CurrProblPediatrAdolesc Health Care 41: 158-176.
- Barker DJ (2004) The developmental origins of adult disease. J Am CollNutr 23: 588S-595S.
- Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ (2002) Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA 288: 728-737.
- Ochiai M, Ichiyama M, Iwayama M, Sakai Y, Yoshida K, et al. (2015) Longitudinal study of very low birth weight infants until 9 years of age; attention deficit hyperactivity and autistic features are correlated with their cognitive functions. Early Hum Dev 91:783-786.
- Aarnoudse-Moens CS, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J (2009) Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics 124: 717-728.
- Indredavik MS, Vik T, Evensen KA, Skranes J, Taraldsen G, et al. (2010) Perinatal risk and psychiatric outcome in adolescents born preterm with very low birth weight or term small for gestational age. J DevBehavPediatr 31: 286-294.
- Pyhälä R, Hovi P, Lahti M, Sammallahti S, Lahti J, et al. (2014) Very low birth weight, infant growth, and autism-spectrum traits in adulthood. Pediatrics 134: 1075-1083.
- Tomi M, Zhao Y, Thamotharan S, Shin BC, Devaskar SU (2013) Early life nutrient restriction impairs blood-brain metabolic profile and neurobehavior predisposing to Alzheimer’s disease with aging. Brain Res 1495: 61-75.
- Akitake Y, Katsuragi S, Hosokawa M,Mishima K, Ikeda T, et al. (2015) Moderate maternal food restriction in mice impairs physical growth, behavior, and neurodevelopment of offspring. Nutr Res 35: 76-87.
- Cai L, Wan CL, He L, de Jong S, Chou KC (2015) Gestational Influenza Increases the Risk of Psychosis in Adults. Med Chem 11: 676-682.
- Parboosing R, Bao Y, Shen L, Schaefer CA, Brown AS (2013) Gestational influenza and bipolar disorder in adult offspring. JAMA Psychiatry 70: 677-685.
- Meyer U (2014) Prenatal poly(i:C) exposure and other developmental immune activation models in rodent systems. Biol Psychiatry 75: 307-315.
- Asp L, Holtze M, Powell SB, Karlsson H, Erhardt S (2010) Neonatal infection with neurotropic influenza A virus induces the kynurenine pathway in early life and disrupts sensorimotor gating in adult Tap1-/- mice. Int J Neuropsychopharmacol 13: 475-485.
- Brown AS, Derkits EJ (2010) Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry 167: 261-280.
- Watson CG, Kucala T, Tilleskjor C, Jacobs L (1984) Schizophrenic birth seasonality in relation to the incidence of infectious diseases and temperature extremes. Arch Gen Psychiatry 41: 85-90.
- O'Callaghan E, Gibson T, Colohan HA, Walshe D, Buckley P, et al. (1991) Season of birth in schizophrenia. Evidence for confinement of an excess of winter births to patients without a family history of mental disorder. Br J Psychiatry 158: 764-769.
- Mednick SA, Machon RA, Huttunen MO, Bonett DB (1988) Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch Gen Psychiatry 45: 189-192.
- Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, et al. (2004) Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry 61: 774-780.
- Xiao J, Buka SL, Cannon TD, Suzuki Y, Viscidi RP, et al. (2009) Serological pattern consistent with infection with type I Toxoplasma gondii in mothers and risk of psychosis among adult offspring. Microbes Infect 11: 1011-1018.
- Gutiérrez-Fernández J, Luna Del Castillo J de D, Mañanes-González S, Carrillo-Ávila JA, Gutiérrez B, et al. (2015) Different presence of Chlamydia pneumoniae, herpes simplex virus type 1, human herpes virus 6, and Toxoplasma gondii in schizophrenia: meta-analysis and analytical study. Neuropsychiatr Dis Treat 11: 843-852.
- Mortensen PB, Pedersen CB, Hougaard DM, Nørgaard-Petersen B, Mors O, et al. (2010) A Danish National Birth Cohort study of maternal HSV-2 antibodies as a risk factor for schizophrenia in their offspring. Schizophr Res 122: 257-263.
- Canetta SE, Bao Y, Co MD, Ennis FA, Cruz J, et al. (2014) Serological documentation of maternal influenza exposure and bipolar disorder in adult offspring. Am J Psychiatry 171: 557-563.
- Atladóttir HO, Thorsen P, Østergaard L, Schendel DE, Lemcke S, et al. (2010) Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism DevDisord 40: 1423-1430.
- Betts KS, Salom CL, Williams GM, Najman JM, Alati R (2015) Associations between self-reported symptoms of prenatal maternal infection and post-traumatic stress disorder in offspring: evidence from a prospective birth cohort study. J Affect Disord 175: 241-247.
- Kneeland RE, Fatemi SH (2013) Viral infection, inflammation and schizophrenia. ProgNeuropsychopharmacolBiol Psychiatry 42: 35-48.
- Fortier ME, Luheshi GN, Boksa P (2007) Effects of prenatal infection on prepulse inhibition in the rat depend on the nature of the infectious agent and the stage of pregnancy. See comment in PubMed Commons below Behav Brain Res 181: 270-277.
- Gallagher D, Norman AA, Woodard CL, Yang G, Gauthier-Fisher A, et al. (2013) Transient maternal IL-6 mediates long-lasting changes in neural stem cell pools by deregulating an endogenous self-renewal pathway. Cell Stem Cell 13: 564-576.
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, et al. (2007) The classical complement cascade mediates CNS synapse elimination. Cell 131: 1164-1178.
- Dammann O, Leviton A (1997) Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res 42: 1-8.
- Smith SE, Li J, Garbett K, Mirnics K, Patterson PH (2007) Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci 27: 10695-10702.
- Ellman LM, Deicken RF, Vinogradov S, Kremen WS, Poole JH, et al. (2010) Structural brain alterations in schizophrenia following fetal exposure to the inflammatory cytokine interleukin-8. Schizophr Res 121: 46-54.
- Cai Z, Pan ZL, Pang Y, Evans OB, Rhodes PG (2000) Cytokine induction in fetal rat brains and brain injury in neonatal rats after maternal lipopolysaccharide administration. Pediatr Res 47: 64-72.
- Brown AS, Sourander A, Hinkka-Yli-Salomäki S, McKeague IW, Sundvall J, et al. (2014) Elevated maternal C-reactive protein and autism in a national birth cohort. Mol Psychiatry 19: 259-264.
- Stolp HB, Dziegielewska KM (2009) Review: Role of developmental inflammation and blood-brain barrier dysfunction in neurodevelopmental and neurodegenerative diseases. NeuropatholApplNeurobiol 35: 132-146.
- Severance EG, Gressitt KL, Buka SL, Cannon TD4, Yolken RH2 (2014) Maternal complement C1q and increased odds for psychosis in adult offspring. Schizophr Res 159: 14-19.
- Chu Y, Jin X, Parada I, Pesic A, Stevens B, et al. (2010) Enhanced synaptic connectivity and epilepsy in C1q knockout mice. ProcNatlAcadSci U S A 107: 7975-7980.
- Glynn MW, Elmer BM, Garay PA, Liu XB, Needleman LA, et al. (2011) MHCI negatively regulates synapse density during the establishment of cortical connections. Nat Neurosci 14: 442-451.
- Leviton A, Gressens P (2007) Neuronal damage accompanies perinatal white-matter damage. Trends Neurosci 30: 473-478.
- Fatemi SH, Emamian ES, Kist D, Sidwell RW, Nakajima K, et al. (1999) Defective corticogenesis and reduction in Reelinimmunoreactivity in cortex and hippocampus of prenatally infected neonatal mice. Mol Psychiatry 4: 145-154.
- Ren-Patterson RF, Cochran LW, Holmes A, Sherrill S, Huang S-J, et al. (2005) Loss of brain-derived neurotrophic factor gene allele exacerbates brain monoamine deficiencies and increases stress abnormalities of serotonin transporter knockout mice. J Neurosci Res 79: 756–771.
- Chapleau CA, Larimore JL, Theibert A, Pozzo-Miller L (2009) Modulation of dendritic spine development and plasticity by BDNF and vesicular trafficking: fundamental roles in neurodevelopmental disorders associated with mental retardation and autism. J NeurodevDisord 1: 185-196.
- McDonald CR, Cahill LS, Ho KT, Yang J, Kim H, et al. (2015) Experimental malaria in pregnancy induces neurocognitive injury in uninfected offspring via a C5a-C5a receptor dependent pathway. PLOS Pathog 11: e1005140.
- Upthegrove R, Manzanares-Teson N, Barnes NM (2014) Cytokine function in medication-naive first episode psychosis: a systematic review and meta-analysis. Schizophr Res 155: 101-108.
- Chez MG, Dowling T, Patel PB, Khanna P, Kominsky M (2007) Elevation of tumor necrosis factor-alpha in cerebrospinal fluid of autistic children. PediatrNeurol 36: 361-365.
- Juckel G, Manitz MP, Brüne M, Friebe A, Heneka MT, et al. (2011) Microglial activation in a neuroinflammational animal model of schizophrenia--a pilot study. Schizophr Res 131: 96-100.
- Maynard TM, Sikich L, Lieberman JA, LaMantia AS (2001) Neural development, cell-cell signaling, and the "two-hit" hypothesis of schizophrenia. Schizophr Bull 27: 457-476.
- Giovanoli S, Engler H, Engler A, Richetto J, Voget M, et al. (2013) Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science 339: 1095-1099.
- Krstic D, Madhusudan A, Doehner J, Vogel P, Notter T, et al. (2012) Systemic immune challenges trigger and drive Alzheimer-like neuropathology in mice. J Neuroinflammation 9: 151.
- Remington G (2008) Alterations of dopamine and serotonin transmission in schizophrenia. Prog Brain Res 172: 117-140.
- Baharnoori M, Bhardwaj SK, Srivastava LK (2012) Neonatal behavioral changes in rats with gestational exposure to lipopolysaccharide: a prenatal infection model for developmental neuropsychiatric disorders. Schizophr Bull 38: 444-456.
- Canetta S, Sourander A, Surcel HM, Hinkka-Yli-Salomäki S, Leiviskä J, et al. (2014) Elevated maternal C-reactive protein and increased risk of schizophrenia in a national birth cohort. Am J Psychiatry 171: 960-968.
- Rogerson SJ, Brown HC, Pollina E, Abrams ET, Tadesse E, et al. (2003) Placental tumor necrosis factor alpha but not gamma interferon is associated with placental malaria and low birth weight in Malawian women. Infect Immun 71: 267-270.
- Conroy AL, Liles WC, Molyneux ME, Rogerson SJ, Kain KC (2011) Performance characteristics of combinations of host biomarkers to identify women with occult placental malaria: a case-control study from Malawi. PLoS One 6: e28540.
- https://www.who.int/healthinfo/global_burden_disease/en/
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences