Efficacy and Effectiveness of Rotavirus Vaccine on Incidence of Diarrhoea among Children: A Meta-analysis
Katayi Mwila-Kazimbaya, Samue Bosomprah, Michelo Simuyandi, Caroline C Chisenga, Roma Chilengi and Sody Munsaka
DOI10.21767/2573-0282.100060
Katayi Mwila-Kazimbaya1,2*, Samuel Bosomprah1,3, Michelo Simuyandi1, Caroline C Chisenga1, Roma Chilengi1,4 and Sody Munsaka2
1Center for Infectious Disease Research in Zambia, Lusaka
2Department of Biomedical Sciences, School of Health Sciences, University of Zambia, Lusaka, Zambia
3Department of Biostatistics, School of Public Health, University of Ghana, Legon, Accra, Ghana
4School of Medicine, University of North Carolina at Chapel Hill, North Carolina, U.S
- *Corresponding Author:
- Kazimbaya KM
Centre for Infectious Disease Research in Zambia, Lusaka, Department of Biomedical Sciences, School of Health Sciences, University of Zambia, Lusaka, Zambia
Tel: +26 0211 242257-63
E-mail: katayi.kazimbaya@cidrz.org
Received date: January 18, 2018; Accepted date: January 31, 2018; Published date: February 2, 2018
Citation: Kazimbaya KM, Bosomprah S , Simuyandi M, Chisenga CC, Chilengi R, et al. (2018) Efficacy and Effectiveness of Rotavirus Vaccine on Incidence of Diarrhoea among Children: A Meta-analysis. Pediatric Infect Dis Vol.3, No.1:4.doi: 10.4172/2573-0282.100060
Copyright: © 2018 Kazimbaya KM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Introduction of rotavirus vaccines has resulted in a decrease in rotavirus related mortality and morbidity. We sought to conduct a meta-analysis to estimate the effect of rotavirus vaccine on incidence of diarrhoea.
Methods: The MEDLINE database was searched through PubMed interface using both textword and subject headings (MeSH). The search strategies were [“rotavirus vaccine effectiveness” or “rotavirus vaccine efficacy” or “rotavirus vaccine eff*”]. The reference lists of the most recent studies identified by the search were checked for additional studies (if not already retrieved). We included both randomised trials and observational studies, which investigated the effect of rotavirus vaccine on incidence of diarrhoea.
Results: There was strong evidence of vaccine efficacy (70%) on incidence of diarrhoea (Pooled risk ratio (pRR)=0.30; 95% confidence interval (CI)=(0.24,0.38); p<0.0001), with much lower vaccine efficacy (63%) in low-middle income countries (LMICs) (pRR=0.37; 95% CI=(56,69); p<0.0001). When restricted to severe diarrhoea outcome, we found 74% vaccine efficacy (pRR=0.26; 95% CI=(0.19,0.24); p<0.0001). For vaccine effectiveness in LMICs, we found 53% vaccine effectiveness (pRR=0.47; 95% CI=(0.36, 0.62); p<0.0001) for 1 dose; 61% effectiveness (pRR=0.39; 95% CI=(0.32, 0.47); p<0.0001) for 2 doses and 72% effectiveness (pRR=0.28; 95% CI=(0.14, 0.56); p<0.0001) for 3 doses.
Conclusion: Incomplete dose series had lower vaccine effectiveness than vaccine efficacy in LMICs where health system capacity is low. However, a 3-dose series had similar effectiveness to vaccine efficacy, suggesting that a booster dose could present a potential benefit in LMIC.
Keywords
Meta-analysis; Efficacy; Effectiveness; Rotarix; RotaTeq; Rotavirus vaccines
Introduction
Rotavirus associated diarrhoea has been a key contributor to the morbidity and mortality among children under 5 years of age worldwide [1] and low-middle income countries (LMICs) bear the greater burden. This problem has led to the widespread introduction of Rotarix™ (GlaxosmithKline Biologicals, Belgium) and RotaTeq™ (Merck, USA) vaccines into national immunization programmes. As of December 2017, 93 countries have done so at either national, sub-national or began phased introduction of the vaccines [2]. The World Health Organization (WHO) made this recommendation after randomised clinical trials showed efficacy in high income countries (HICs) of between 80-95% [3-6].
Although subsequent trials from low and middle income countries (LMICs) showed much lower efficacy rates between 40 and 60% [7-10], the public health impact in these high burden settings was still compelling enough to continue the immunization campaigns. Several other vaccines are in the developmental and licensure phase such as Rotavin™ and RotaVac™ which have partial or restricted licensure in China, Vietnam and India [11,12]. Rotasil™ is another vaccine currently under development and was recently tested in Niger [13]. All have shown similar vaccine efficacy and effectiveness trends in LMICs [11-14].
Much data on post licensure effectiveness of rotavirus vaccines has been published on HICs which still indicate how well rotavirus vaccines have worked. LMICs researchers are now also beginning to generate more information on vaccine effectiveness in their respective locations that has shown that vaccine responses have continued to be sub-optimal [7-10,15-17].
We will focus on consolidating vaccine efficacy and effectiveness data for globally licensed vaccines Rotarix and RotaTeq in HICs and LMICs as well as some of the steps and areas that still need to be addressed in order to improve vaccine effectiveness in LMICs.
Methods
Search strategy for identification of studies
The MEDLINE database was searched through PubMed interface using both textword and subject headings (MeSH). The search strategies were [“rotavirus vaccine effectiveness” or “rotavirus vaccine efficacy” or “rotavirus vaccine eff*”], which retrieved 858 studies. The reference lists of the most recent studies identified by the search were checked for additional studies (if not already retrieved).
Criteria for including studies for the review
In the efficacy analysis we included randomised clinical trials reporting efficacy of Rotarix (RV1) and RotaTeq (RV5). In the effectiveness analysis we included observational studies reporting population effectiveness of Rotarix (RV1) or RotaTeq (RV5) against hospital admission for rotavirus gastroenteritis (RVGE) or acute gastroenteritis (AGE) for incomplete and complete doses in all countries regardless of whether they are included in national immunisation programmes or privately offered. Duplicates were removed, as were cost effective, genotype specific, impact, methodological and review articles, leaving 68 articles for inclusion in the analysis. Efficacy data included overall efficacy and severe rotavirus related gastroenteritis, whilst effectiveness data included efficacy against hospital admission. All studies published until October 2017 was eligible for inclusion.
Data extraction
Two authors (K.M-K, SB) extracted data from the studies using data extraction form designed to capture relevant data for this purpose and the differences (if any) were reconciled. For the efficacy studies, the measure of effect for the meta-analysis was risk ratio (RR), which measures cumulative incidence more accurately. For studies that reported odds ratio, we recalculated risk ratios by reconstructing the 2×2 tables from data in the original paper because odds ratios usually overestimate risk ratios especially where the incidence of the outcome is common (>10%).
The recalculation was convenient because efficacy studies are required to report the unadjusted result as primary analysis [18]. For the effectiveness studies, we did not recalculate the measure of effect because the primary analysis was adjusted effect. All studies measured diarrhoea severity using Vesikari scores of 11 [19] or Clarke score of 16 [20]. We followed the preferred reporting items for systematic reviews and metaanalysis (PRISMA) in the conduct of this review. Severe diarrhoea was defined as Vesikari score of 11 or greater. Any diarrhoea was defined as mild or moderate or severe.
Statistical analysis
We calculated a weighted average of the effect measures across studies using ‘metan’ command in Stata. Forest plot was also presented. The ‘metan’ command is flexible for any measure of effect because it requires either the frequencies of events in exposed and unexposed group (the approach we used in the efficacy studies) or the logarithm of the effect measure and its standard error (the approach we used in the effectiveness studies). For studies that reported zero events, we replaced the zeros with 0.5 before performing the meta-analysis. For the effectiveness studies, we calculated the standard error of the log-risk ratio or log-odds ratio by back-transforming the relevant confidence intervals as reported in the papers. Studies conducted in different epidemiological settings are likely to vary, so we performed a chi-squared test of heterogeneity.
If there was evidence of heterogeneity, the individual study effect estimates were combined using random effects metaanalysis, which incorporates between-study variability in the weighting. P-values less than 0.05 were considered to show strong evidence of association. Published studies may not be representative of all valid studies undertaken and this can bias meta-analysis. We assessed publication bias using Harbord's modified test for small-study effects [21]. All analyses were performed using Stata 15 MP (StatCorp, College Station, TX, USA).
Results
Overview of included studies
The search identified a total of 858 studies out of which 228 were duplicates (Figure 1). Of the 630 non-duplicated studies, we excluded 98 cost-effectiveness, 67 genotype specific, 13 impact, 21 reviews, and 7 methodological studies leaving a total of 424 studies. After applying the eligibility criteria, we further excluded 357 studies leaving a total of 67 full text articles for analysis out of which 16 were severe RVGE efficacy, 4 overall efficacy, and 47 efficacy against hospital admission studies (Figure 1).
Characteristics of included studies
27 studies investigated the efficacy of rotavirus vaccine on incidence of diarrhoea, out of which 7 studies investigated the overall efficacy of the vaccines [22-31] while 20 studies investigated efficacy on severe diarrhoea (Table 1) [4,5,31-45]. 23 studies were in LMICs while 4 studies were in HICs contributing a total sample size of 135,486 (Table 1). Data from the HICs included data from multicenter trials that was not disaggregated by country. 13 investigated RV1 and 6 investigated RV5 (Table 1).
| Reference | Country/Region | Demo | Vaccine Type | Sample Size | Loss to Follow Up | Study Duration (months) | Efficacy | Vaccinated No Diarrhoea | Vaccinated Diarrhoea | Non- Vaccinated No Diarrhoea | Non- Vaccinated Diarrhoea |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Arajuo et al. [82] | Brazil | LMIC | RV1 | 2155 | 1 | 8 | 64.5 | 460 | 22 | 130 | 19 |
| Armah et al. [10] | Ghana, Mali and Kenya | LMIC | RV5 | 5468 | 134 | 17.5 | 64.2 | 2712 | 21 | 2735 | 58 |
| Armah et al. [10] | Ghana | LMIC | RV5 | 5468 | 134 | 18.2 | 65 | 3255 | 15 | 3235 | 42 |
| Armah et al. [10] | Mali | LMIC | RV5 | 5468 | 134 | 18.2 | 1 | 2835 | 4 | 2844 | 4 |
| Armah et al. [10] | Kenya | LMIC | RV5 | 5468 | 134 | 16 | 83.4 | 1892 | 2 | 1876 | 12 |
| Bhandari et al. [26] | India | LMIC | Rotavac | 4532 | 101 | 20 | 56.3 | 4298 | 56 | 2123 | 64 |
| Cunliffe et al.[27] | Malawi | LMIC | RV1 | 9 | 19 | 49.7 | 989 | 41 | 445 | 38 | |
| Feikin et al. [33] | Kenya | LMIC | RV5 | 1308 | 185 | 7 | 83.4 | 569 | 2 | 552 | 12 |
| Iwata et al. [28] | Japan | HIC | RV5 | 762 | 4 | 7 | 100 | 355 | 0 | 346 | 10 |
| Lau et al. [31] | China, Hong Kong | LMIC | RV1 | 3025 | 20 | 100 | 1494 | 0 | 1491 | 8 | |
| Li et al. [32] | China | LMIC | RV1 | 3333 | 13 | 24 | 75 | 1567 | 8 | 1541 | 32 |
| Linhares et al. [32] | Latin America | LMIC | RV1 | 14286 | 248 | 20 | 83.1 | 7195 | 10 | 7023 | 58 |
| Madhi et al. [94] | SA and Malawi | LMIC | RV1 | 4939 | 206 | 12 | 61.2 | 2918 | 56 | 1373 | 70 |
| Madhi et al. [94] | South Africa | LMIC | RV1 | 973 | 206 | 12 | 81.5 | 1929 | 15 | 928 | 32 |
| Madhi et al. [94] | Malawi | LMIC | RV1 | 505 | 206 | 12 | 49.7 | 989 | 41 | 445 | 38 |
| Mo et al. [35] | China | LMIC | RV5 | 4040 | 1 | 12 | 78.9 | 1916 | 11 | 1885 | 52 |
| Phua et al. [29,31] | Hong Kong, Singapore and Taiwan | HIC | RV1 | 10519 | 155 | 21 | 96.1 | 5261 | 1 | 5205 | 51 |
| Ruiz-Palacios et al. [4] | Latin America | LMIC | RV1 | 20169 | 239 | 12 | 84.8 | 8997 | 12 | 8781 | 77 |
| Sow et al. [22] | Mali | LMIC | RV5 | 1960 | 36 | 12 | 1 | 782 | 41 | 754 | 71 |
| Tregnaghi et al. [38] | Latin America | LMIC | RV1 | 6568 | 34 | 7.4 | 81.6 | 4204 | 7 | 2080 | 19 |
| Vesikari et al. [101] | Europe | HIC | RV1 | 3994 | 23 | 5.7 | 95.8 | 2567 | 5 | 1242 | 60 |
| Vesikari et al. [101] | European | HIC | RV1 | 3874 | 23 | 5.7 | 95.7 | 2568 | 4 | 1254 | 48 |
| Zaman et al. [25] | Bangladesh and Vietnam | LMIC | RV5 | 2036 | 7 | 21 | 51 | 953 | 38 | 907 | 71 |
| Zaman et al. [25] | Bangladesh | LMIC | RV5 | 7 | 21 | 45.7 | 953 | 17 | 907 | 31 | |
| Zaman et al. [25] | Vietnam | LMIC | RV5 | 7 | 21 | 72.3 | 953 | 2 | 907 | 7 | |
| Zaman et al. [25] | Bangladesh | LMIC | RV1 | 12318 | 42.8 | 5784 | 53 | 5066 | 101 | ||
| Zaman et al. [25] | Bangladesh | LMIC | RV1 | 12318 | 7 | 24 | 41.5 | 5735 | 102 | 4995 | 172 |
Table 1: Features of studies included in vaccine efficacy analysis
47 studies investigated vaccine effectiveness (Table 2) [5-16,40-77]. 29 studies were from HICs [5,47,48,60-62,72-74,78-84] and 18 were from LMICs (Table 2) [7-16,40-47,50-54,67-79]. 20 studies evaluated RV1, 10 studies evaluated RV5 and 11 studies evaluated the use of either RV1 or RV5. The total sample size across all the studies was 777,809 (Table 2).
| Reference | Country/ Region | Demo | Sample Size | Vaccine Type |
|---|---|---|---|---|
| Abeid et al. [52] | Zanzibar | LMIC | 805 | RV1 |
| Adlhoch et al. [59] | Germany | HIC | 368 | RV1/RV5 |
| Araki et al. [65] | Japan | HIC | RV1/RV5 | |
| Armah et al. [101] | Ghana | LMIC | 657 | RV1 |
| Beres et al. [7] | Zambia | LMIC | 529 | RV1 |
| Boom et al. [48] | USA | HIC | 205 | RV5 |
| Braeckman et al. [45] | Belgium | HIC | 431 | RV1 |
| Cardellino et al. [75] | Nicaragua | LMIC | RV5 | |
| Castilla et al. [57] | Spain | HIC | 6792 | RV1/RV5 |
| Chang et al. [55] | China | HIC | 1088 | RV1 |
| Chang et al. [55] | China | HIC | RV5 | |
| Cortese et al. [44] | USA- RV1 | HIC | 593 | RV1 |
| Cortese et al. [44] | USA-RV5 | HIC | 593 | RV5 |
| Cotes-Cantillo et al. [42] | Colombia | LMIC | 974 | RV1 |
| de Palma et al. [40] | El Salvador | LMIC | 323 | RV1 |
| Desai et al. [63] | USA | HIC | 122 | RV1/RV5 |
| Field et al. [72] | Australia | HIC | 459 | RV5 |
| Fujii et al. [62] | Japan | HIC | 244 | RV1/RV5 |
| Gastanaduy et al. [83] | Botswana | LMIC | 610 | RV1 |
| Gastanaduy et al. [83] | Guatemala | LMIC | 1417 | RV1/RV5 |
| Gheorgita et al. [85] | Moldova | LMIC | 957 | RV1 |
| Gosselin et al. [60] | Canda | HIC | 11203 | RV1/RV5 |
| Groome et al. [81] | South Africa | LMIC | 1974 | RV1 |
| Ichihara et al. [46] | Brazil | LMIC | 2176 | RV1 |
| Justino et al. [54] | Brazil | LMIC | 1045 | RV1 |
| Leshem et al. [69] | Israel | HIC | 515 | RV5 |
| Marlow et al. [64] | Portugal | HIC | RV1/RV5 | |
| Martinon-Torres et al. [58] | Spain | HIC | 467 | RV1/RV5 |
| Mast et al. [71,73] | Nicaragua | LMIC | 1092 | RV5 |
| Muhsen et al. [53] | Israel | HIC | 327 | RV1 |
| Patel et al. [67] | Nicaragua | LMIC | 975 | RV5 |
| Patel et al. [67] | Bolivia | LMIC | 2318 | RV1 |
| Payne et al. [68] | USA | HIC | 904 | RV1 |
| Payne et al. [68] | USA | HIC | 2961 | RV5 |
| Perez-Vilar et al. [80] | Spain | HIC | 174744 | RV1 |
| Perez-Vilar et al. [80] | Spain | HIC | 174744 | RV5 |
| Pringle et al. [10] | Bolivia | LMIC | 776 | RV1 |
| Sehakyan et al. [81] | Aremenia | LMIC | 486 | RV1 |
| Snelling et al. [41] | Australia | HIC | 208 | RV1 |
| Staat et al.[68] | USA | HIC | 833 | RV5 |
| Tate et al. [84] | Rwanda | LMIC | 200 | RV5 |
| Tharmaphornpilas et al. [61] | Thailand | HIC | 2893 | RV1/RV5 |
| Vesikari et al. [101] | Finland | HIC | 509 | RV5 |
| Wang et al. [71] | USA | HIC | 59307 | RV5 |
| Wang et al. [71] | USA | HIC | 146237 | RV5 |
| Yang et al. [76] | Taiwan | HIC | 201 | |
| Yeung et al. [66] | Japan | HIC | 404 | RV1/RV5 |
Table 2: Features of studies included in vaccine effectiveness analysis.
Vaccine efficacy on incidence of diarrhoea (severe and/or any)
There was evidence of substantial variability between studies (I2=74.4%, p<0.0001) with about 74.4% of the pooled betweenstudy heterogeneity attributable to the variability in the true effect (Figure 2A). There was strong evidence of vaccine efficacy (70%) on incidence of diarrhoea (Pooled risk ratio (pRR)=0.30; 95% confidence interval (CI)=(0.24, 0.38); p<0.0001) (Figure 2A).
Figure 2a: Efficacy of rotavirus vaccine on incidence of diarrhoea (severe and any) by country income status. ES represents the estimated risk ratio, I2 is a measure of heterogeneity between studies. LMIC represents low and middle income countries and HIC represents high income countries. RV1=Rotarix and RV5=RotaTeq.
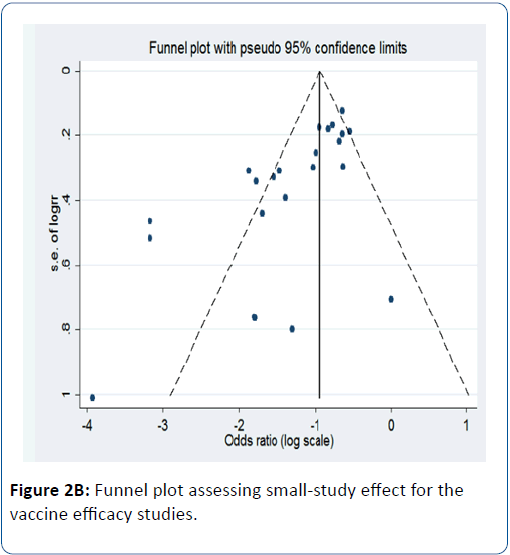
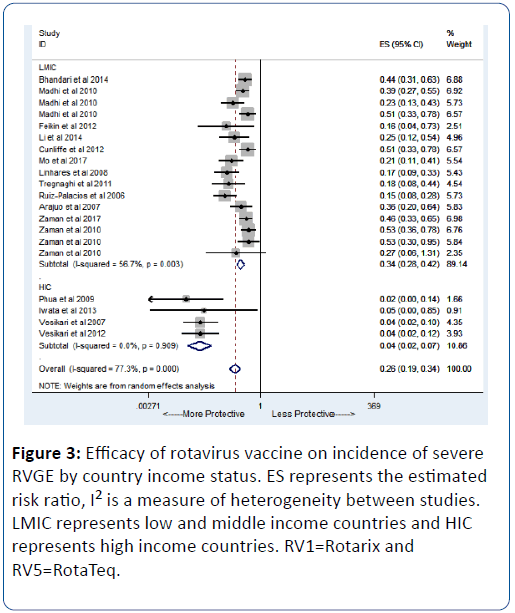
When stratified by country income status, we observed strong evidence of vaccine efficacy (96%) in HICs (pRR=0.04; 95% CI=(93, 98); p<0.0001) while an efficacy of 63% was observed in LMICs (pRR=0.37; 95% CI=(56, 69); p<0.0001) (Figure 2A). We observed evidence of publication bias in terms of small-study effect (bias=-2.55; Harbord’s modified test p=0.017) (Figure 2B). When restricted to severe diarrhoea as the outcome, we also found strong evidence of vaccine efficacy (74%) (pRR=0.26; 95% CI=(0.19,0.24); p<0.0001) (Figure 3). For any diarrhoea outcome, the efficacy was 53% (pRR=0.47; 95% CI=(0.36,0.60); p<0.0001) (Figure 4).
Figure 3: Efficacy of rotavirus vaccine on incidence of severe RVGE by country income status. ES represents the estimated risk ratio, I2 is a measure of heterogeneity between studies. LMIC represents low and middle income countries and HIC represents high income countries. RV1=Rotarix and RV5=RotaTeq.
Figure 4: Efficacy of rotavirus vaccine on incidence of any diarrhoea by country income status. ES represents the estimated risk ratio, I2 is a measure of heterogeneity between studies. LMIC represents low and middle income countries and HIC represents high income countries. RV1=Rotarix and RV5=RotaTeq.
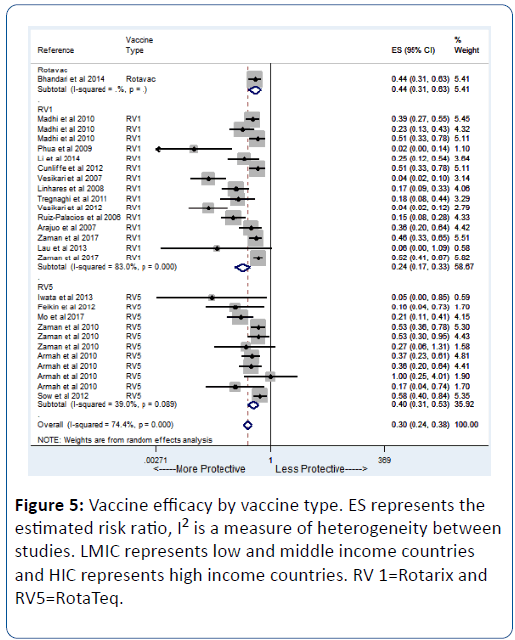
In a secondary analysis to assess the efficacy of specific vaccine type, we found similar results. RV1 showed an efficacy of 76% (pRR=0.24; 95% CI=(0.17, 0.33) while RV5 showed an efficacy of 60% (pRR=0.40; 95% CI=(0.31, 0.53) (Figure 5).
Vaccine effectiveness on incidence of diarrhoea
In assessing the effect of rotavirus vaccine in real world setting, we found strong evidence of about 78% reduction in incidence of diarrhoea due to the vaccine (pRR=0.22; 95% CI=(0.18, 0.28); p<0.0001).
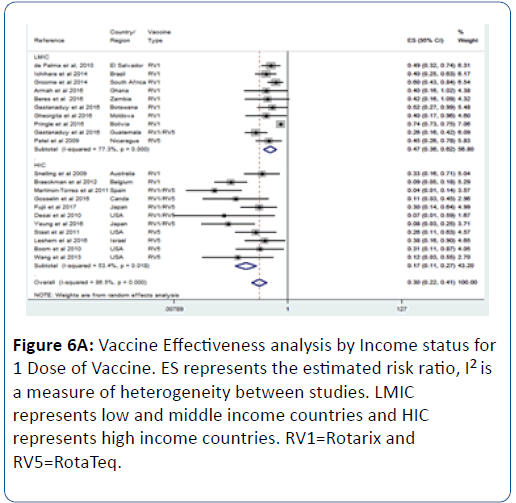
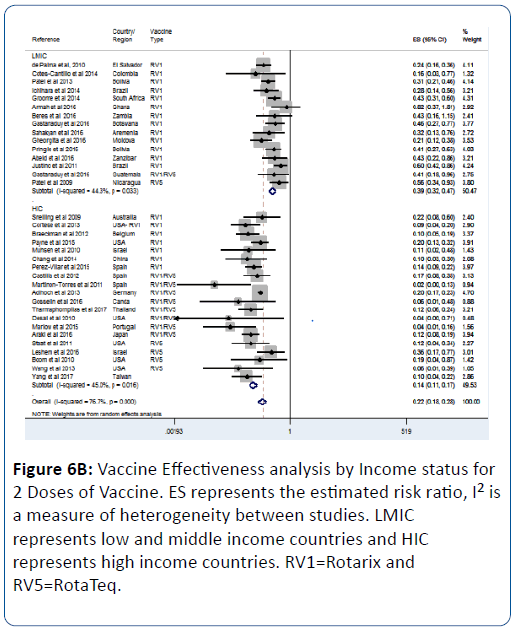
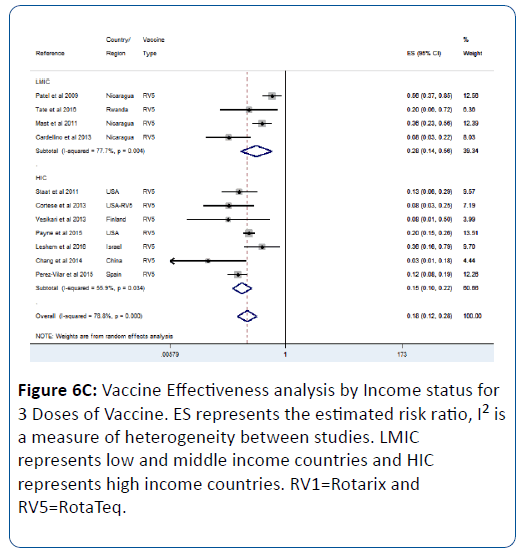
This effect was influenced by the number of vaccine doses; we observed that as the number of dose increases so does the vaccine effectiveness (Figures 6A-6C). For 1 vaccine dose, the vaccine effectiveness in LMIC was 53% (pRR=0.47; 95% CI=(0.36, 0.62); p<0.0001) (Figure 6A). For 2 doses, the effectiveness in LMIC was 61% (pRR=0.39; 95% CI=(0.32, 0.47); p<0.0001) (Figure 6B). For 3 doses, the effectiveness in LMIC was 72% (pRR=0.28; 95% CI=(0.14, 0.56); p<0.0001) (Figure 6C).
Discussion
Results from both the efficacy and effectiveness trials show that rotavirus vaccines have been effective in reducing the scourge of rotavirus associated diarrhoea. Our analysis was able to consolidate data that shows HICs have consistently higher efficacy and effectiveness rates than LMICs; and is true for both RV1 and RV5.
Effectiveness data from real world setting results have also indicated that incomplete vaccine series are able to provide some protection to infants though to a lesser extent than a complete series. The incomplete series had a much lower effectiveness in LMIC than vaccine efficacy in LMIC. Incomplete series is common in LMICs where inadequate health facilities and long distances to health facilities exist. We found that a 3- dose vaccine series had effect similar to vaccine efficacy in LMIC, making it a logical argument for a booster dose especially in LMICs.
Improved vaccine effectiveness: Terrain à forte
The differences in efficacy and effectiveness in HICs and LMICs however still remain the main discussion point as there is need for further reduction in rotavirus associated mortality and morbidity [85]. Various factors have been postulated as contributing to this observed effect. Host factors such as genetics, malnutrition, enteric environmental dysfunction (EED), maternal factors such as antibodies passed onto the infant, various components of breast milk, exposure to HIV and other environmental factors including poor sanitation, concurrent infection with other pathogens have all been postulated to influence vaccine effectiveness [86-97].
Another factor postulated to possibly have an effect is strain diversity in HICs and LMICs. Both RV1 and RV5 are vaccines originally designed by HIC researchers from strains present in HIC regions. However, research has indicated that the vaccines are not strain specific but cross-cutting without evidence of vaccine induced selection pressure [43,98,99]. Nonetheless, the increased strain diversity being observed in many LMICs requires seroepidemiological vigilance to ensure tracking of any emerging strains such as P[4]G2 that may account for reduced efficacy through limited cross protection [100-104].
Despite greater understanding of contributing factors to reduced vaccine efficacy, we are faced with the fact that a lot of these factors are almost impossible to resolve. The high maternal immunity that is passed onto the child is a consequence of where one lives and poor water, sanitation and hygiene (WASH) that cannot easily be changed. Unless this is addressed, mothers will continue to pass these, on to their infants. The same applies to the EED; in order to change the micro biome that exists in individuals, it will require interventions that address which organisms are first introduced into the system. Additionally in low income settings with low availability of funds, the use of formula is not a feasible solution to address the issue of maternal antibodies passed onto the child during breast feeding.
Genetic makeup has also been included in the list of factors affecting vaccine effectiveness. Despite advances in science, genetics is still a growing area and we have not yet reached a point where we can change ones genetic code if one is predisposed towards a disease even in the developed settings. Thus, genetic predisposition is another unsolvable in the quest for better vaccine effectiveness. Another key area is that of mal(nutrition) in LMICs. Again, despite great efforts being made worldwide, the magnitude of this problem renders it unsolvable for years to come. Unless we can find a world in which all children are able to have sufficient food and the right kind of food, this too shall remain a hindrance to our efforts to obtain better vaccine effectiveness.
Future perspectives
Despite the many hindrances to achieving better vaccine effectiveness in LMICs there are still many other areas that are available to work on. The next generation of vaccines can be targeted to areas that maybe within our control such as alternative routes of administration; short course full dose regimen. The ease of use and lower cost of oral vaccines is the main reason for their inclusion in national immunisation programmes. However, the large number of interfering factors has led us to reassess their use. As is the case with Polio, we may have to go the parenteral route to effectively circumvent the problems encountered via the oral route [105].
Another way of dealing with interference is the adjustment of the vaccine schedule; a neonatal dosing schedule has been proposed as potentially beneficial to improved vaccine immunogenicity [106,107]. A booster dose has also been proposed as viable option for enhanced vaccine immune responses of current vaccines in use [108]. While use of the expanded programme on immunization (EPI) was recommended in order to reach as many infants as possible, there may be larger benefits in offering immunization options outside of this schedule. This could be in the form of changing the time to one at which maternal antibodies are waning or as early as possible to ensure adequate protection from early exposure. Nonetheless, these options need to be weighed against the challenge of low coverage in LMIC [109], when venturing outside of the EPI.
Lastly, use of effective adjuvants has not been fully explored in rotavirus immunology [110]. This is particularly key when considering neonates in whom the immune system is naïve [111,112]; and yet it’s a practical window to beat the early exposure of infants to pathogens [88].
Conclusion
While current rotavirus vaccines have saved many lives in LMIC settings, there are still clear gaps in vaccine performance. This paper has comprehensively shown the differences and need for concerted effort to improve vaccine performance in these areas where in fact, vaccines are most needed.
References
- Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, et al. (2013) Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. The Lancet 382: 209-222.
- Global Introduction Status, Rota Council.
- Vesikari T, Karvonen A, Puustinen L, Zeng SQ, Szakal ED, et al. (2004) Efficacy of RIX4414 live attenuated human rotavirus vaccine in Finnish infants. Pediatr Infect Dis J 23: 937-943.
- Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, Abate H, Breuer T, et al. (2006) Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 354: 11-22.
- Vesikari T, Prymula R, Schuster V, Tejedor JC, Cohen R, et al. (2012) Efficacy and immunogenicity of live-attenuated human rotavirus vaccine in breast-fed and formula-fed European infants. Pediatr Infect dis J 31: 509-513.
- WHO (2013) Rotavirus Vaccines WHO Position Paper-January 2013. Wkly Epidemiol Rep 88: 49-64.
- Beres LK, Tate JE, Njobvu L, Chibwe B, Rudd C, et al. (2016) A preliminary assessment of rotavirus vaccine effectiveness in Zambia. Clin Infect Dis 62: S175-S182.
- Bar-Zeev N, Tate JE, Pecenka C, Chikafa J, Mvula H, et al. (2016) Cost-effectiveness of monovalent rotavirus vaccination of infants in Malawi: a postintroduction analysis using individual patient–level costing data. Clin Infect Dis 62: S220-S228.
- Gastañaduy PA, Steenhoff AP, Mokomane M, Esona MD, Bowen MD, et al. (2016) Effectiveness of monovalent rotavirus vaccine after programmatic implementation in Botswana: a multisite prospective case-control study. Clin Infect Dis 62: S161-S167.
- Armah G, Pringle K, Enweronu-Laryea CC., Ansong D, Mwenda JM, et al. (2016) Impact and effectiveness of monovalent rotavirus vaccine against severe rotavirus diarrhea in Ghana. Clin Infect Dis 62: S200-S207.
- Anh DD, Van Trang N, Thiem VD, Anh NTH, Mao ND, et al. (2012) A dose-escalation safety and immunogenicity study of a new live attenuated human rotavirus vaccine (Rotavin-M1) in Vietnamese children. Vaccine 30: A114-A121.
- Fu C, He Q, Xu J, Xie H, Ding P, et al. (2012) Effectiveness of the Lanzhou lamb rotavirus vaccine against gastroenteritis among children. Vaccine 31: 154-158.
- Isanaka S, Guindo O, Langendorf C, Matar Seck A, Plikaytis BD, et al. (2017) Efficacy of a low-cost, heat-stable oral rotavirus vaccine in Niger. N Engl J Med 376: 1121-1130.
- O’Ryan M (2017) Rotavirus Vaccines: a story of success with challenges ahead. F1000 Research 6: 1517.
- Enane LA, Gastañaduy PA, Goldfarb DM, Pernica JM, Mokomane M, et al. (2016) Impact of rotavirus vaccination on hospitalizations and deaths from childhood gastroenteritis in Botswana. Clin Infect Dis 62: S168-S174.
- Enane LA, Gastañaduy PA, Goldfarb DM, Pernica JM, Mokomane M, et al. (2016) Impact of rotavirus vaccination on hospitalizations and deaths from childhood gastroenteritis in Botswana. Clin Infect Dis 62: S168-S174.
- Bar-Zeev N, Jere KC, Bennett A, Pollock L, Tate JE, et al. (2016) Population impact and effectiveness of monovalent rotavirus vaccination in urban Malawian children 3 years after vaccine introduction: ecological and case-control analyses. Clin Infect Dis 62: S213-S219.
- Schulz KF, Altman DG, Moher D, CONSORT Group (2010) CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med 8: 18.
- Ruuska T, Vesikari T (1990) Rotavirus Disease in Finnish Children: Use of Numerical Scores for Clinical Severity of Diarrhoeal Episodes. Scand J Infect Dis 22: 259-267.
- Clark HF, Horian FE, Bell LM, Modesto K, Gouvea V, et al. (1988) Protective effect of WC3 vaccine against rotavirus diarrhea in infants during a predominantly serotype 1 rotavirus season. J Infect Dis 158: 570-587.
- Harbord RM, Egger M, Sterne JAC (2006) A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med 25: 3443-3457.
- Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, et al. (2010) Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. The Lancet 376: 606-614.
- Lau YL, Nelson EAS, Poon KH, Chan PK, Chiu S, et al. (2013) Efficacy, safety and immunogenicity of a human rotavirus vaccine (RIX4414) in Hong Kong children up to three years of age: a randomized, controlled trial. Vaccine 31: 2253-2259.
- Sow SO, Tapia M, Haidara FC, Ciarlet M, Diallo F, et al. (2012) Efficacy of the oral pentavalent rotavirus vaccine in Mali. Vaccine 30: A71-A78.
- Zaman K, Sack DA, Neuzil KM, Yunus M, Moulton LH, et al. (2017) Effectiveness of a live oral human rotavirus vaccine after programmatic introduction in Bangladesh: A cluster-randomized trial. PLoS medicine 14: e1002282.
- Bhandari N, Rongsen-Chandola T, Bavdekar A, John J, Antony K, et al. (2014) Efficacy of a monovalent human-bovine (116E) rotavirus vaccine in Indian infants: a randomised, double-blind, placebo-controlled trial. The Lancet 383: 2136-2143.
- Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten M, et al. (2010) Effect of human rotavirus vaccine on severe diarrhoea in African infants. New Engl J Med 362: 289-298.
- Iwata S, Nakata S, Ukae S, Koizumi Y, Morita Y, et al. (2013) Efficacy and safety of pentavalent rotavirus vaccine in Japan: a randomized, double-blind, placebo-controlled, multicenter trial. Hum vaccines immunother 9: 1626-1633.
- Phua KB, Lee BW (2016) Efficacy, Immunogenicity and Safety of a Human Rotavirus Vaccine RIX4414 in Singaporean Infants. Ann Acad Med Singapore 45: 44-50.
- Zaman K, Anh DD, Victor JC, Shin S, Yunus M, et al. (2010) Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. The Lancet 376: 615-623.
- Phua KB, Lim FS, Lau YL, Nelson EAS, Huang LM, et al. (2009) Safety and efficacy of human rotavirus vaccine during the first 2 years of life in Asian infants: randomised, double-blind, controlled study. Vaccine 27: 5936-5941.
- Li RC, Huang T, Li YP, Luo D, Tao J, et al. (2014) Human rotavirus vaccine (RIX4414) efficacy in the first two years of life: a randomized, placebo-controlled trial in China. Hum vaccines immunother 10: 11-18.
- Feikin DR, Laserson KF, Ojwando J, Nyambane G, Ssempijja V, et al. (2012) Efficacy of pentavalent rotavirus vaccine in a high HIV prevalence population in Kenya. Vaccine 30: A52-A60.
- Cunliffe NA, Witte D, Ngwira BM, Todd S, Bostock NJ, et al. (2012) Efficacy of human rotavirus vaccine against severe gastroenteritis in Malawian children in the first two years of life: a randomized, double-blind, placebo controlled trial. Vaccine 30: A36-A43.
- Mo Z, Mo Y, Li M, Tao J, Yang X, et al. (2017) Efficacy and safety of a pentavalent live human-bovine reassortant rotavirus vaccine (RV5) in healthy Chinese infants: A randomized, double-blind, placebo-controlled trial. Vaccine 35: 5897-5904.
- Vesikari T, Karvonen A, Prymula R, Schuster V, Tejedor JC, et al. (2007) Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. The Lancet 370: 1757-1763.
- Linhares AC, Velázquez FR, Pérez-Schael I, Sáez-Llorens X., Abate H, et al. (2008) Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. The Lancet 371: 1181-1189.
- Tregnaghi MW, Abate HJ, Valencia A, Lopez P, Da Silveira TR, et al. (2011) Human rotavirus vaccine is highly efficacious when coadministered with routine expanded program of immunization vaccines including oral poliovirus vaccine in Latin America. Pediatr Infect Dis J 30: e103-e108.
- Araujo EC, Clemens SAC, Oliveira CS, Justino MCA, Rubio P, et al. (2007) Safety, immunogenicity, and protective efficacy of two doses of RIX4414 live attenuated human rotavirus vaccine in healthy Brazilian infants. Jornal de pediatria 83: 217-224.
- de Palma O, Cruz L, Ramos H, de Baires A, Villatoro N, et al. (2010) Effectiveness of rotavirus vaccination against childhood diarrhoea in El Salvador: case-control study. BMJ 340: c2825.
- Snelling TL, Schultz R, Graham J, Roseby R, Barnes GL, et al. (2009) Rotavirus and the indigenous children of the Australian outback: monovalent vaccine effective in a high-burden setting. Clin Infect Dis 49: 428-431.
- Cotes-Cantillo K, Paternina-Caicedo A, Coronell-Rodríguez W, Alvis-Guzmán N, Parashar UD, et al. (2014) Effectiveness of the monovalent rotavirus vaccine in Colombia: a case-control study. Vaccine 32: 3035-3040.
- Patel MM, Patzi M, Pastor D, Nina A, Roca Y, et al. (2013) Effectiveness of monovalent rotavirus vaccine in Bolivia: case-control study. BMJ 346: f3726.
- Cortese MM, Immergluck LC, Held M, Jain S, Chan T, et al. (2013) Effectiveness of monovalent and pentavalent rotavirus vaccine. Pediatrics 132: e25-33.
- Braeckman T, Van Herck K, Meyer N, Pirçon JY, Soriano-Gabarró M, et al. (2012). Effectiveness of rotavirus vaccination in prevention of hospital admissions for rotavirus gastroenteritis among young children in Belgium: case-control study. BMJ 345: e4752.
- Ichihara MY, Rodrigues LC, Santos CAT, Maria da Gloria LC, De Jesus SR, et al. (2014) Effectiveness of rotavirus vaccine against hospitalized rotavirus diarrhea: a case–control study. Vaccine 32: 2740-2747.
- Groome MJ, Page N, Cortese MM, Moyes J, Zar HJ, et al. (2014) Effectiveness of monovalent human rotavirus vaccine against admission to hospital for acute rotavirus diarrhoea in South African children: a case-control study. Lancet Infect Dis 14: 1096-1104.
- Payne DC, Selvarangan R, Azimi PH, Boom JA, Englund JA, et al. (2015) Long-term consistency in rotavirus vaccine protection: RV5 and RV1 vaccine effectiveness in US children, 2012–2013. Clin Infect Dis 61: 1792-1799.
- Sahakyan G, Grigoryan S, Wasley A, Mosina L, Sargsyan S, et al. (2016) Impact and effectiveness of monovalent rotavirus vaccine in Armenian children. Clin Infect Dis 62: S147-S154.
- Gheorghita S, Birca L, Donos A, Wasley A, Birca I, et al. (2016) Impact of rotavirus vaccine introduction and vaccine effectiveness in the Republic of Moldova. Clin Infect Dis 62: S140-S146.
- Pringle KD, Patzi M, Tate JE, Iniguez Rojas V, Patel M, et al. (2016) Sustained effectiveness of rotavirus vaccine against very severe rotavirus disease through the second year of life, Bolivia 2013-2014. Clin Infect Dis 62: S115-S120.
- Abeid KA, Jani B, Cortese MM, Kamugisha C, Mwenda JM, et al. (2016) Monovalent rotavirus vaccine effectiveness and impact on rotavirus hospitalizations in Zanzibar, Tanzania: data from the first 3 years after introduction. J Infect Dis 215: 183-191.
- Muhsen K, Shulman L, Kasem E, Rubinstein U, Shachter J, et al. (2010) Effectiveness of rotavirus vaccines for prevention of rotavirus gastroenteritis-associated hospitalizations in Israel: a case-control study. Human vaccines 6: 450-454.
- Justino MCA, Linhares AC, Lanzieri TM, Miranda Y, Mascarenhas JDA, et al. (2011) Effectiveness of the monovalent G1P [8] human rotavirus vaccine against hospitalization for severe G2P [4] rotavirus gastroenteritis in Belem, Brazil. Pediatr Infect Dis J 30: 396-401.
- Chang WC, Yen C, Wu FT, Huang YC, Lin JS, et al. (2014) Effectiveness of 2 rotavirus vaccines against rotavirus disease in Taiwanese infants. Clin Infect Dis 33: e81-e86.
- Pérez-Vilar S, Díez-Domingo J, López-Lacort M, Martínez-Úbeda S, Martinez-Beneito MA (2015) Effectiveness of rotavirus vaccines, licensed but not funded, against rotavirus hospitalizations in the Valencia Region, Spain. BMC Infect Dis 15: 92.
- Castilla J, Beristain X, Martínez-Artola V, Navascués A, Cenoz MG, et al. (2012) Effectiveness of rotavirus vaccines in preventing cases and hospitalizations due to rotavirus gastroenteritis in Navarre, Spain. Vaccine 30: 539-543.
- Martinon-Torres F, Alejandro MB, Collazo LR, Lastres JMS, Díaz SP, et al. (2011) Effectiveness of rotavirus vaccination in Spain. Human vaccines 7: 757-761.
- Adlhoch C, Hoehne M, Littmann M, Marques AM, Lerche A, et al. (2013) Rotavirus vaccine effectiveness and case-control study on risk factors for breakthrough infections in Germany, 2010–2011. Pediatr Infect Dis J 32: e82-e89.
- Gosselin V, Généreux M, Gagneur A, Petit G,(2016) Effectiveness of rotavirus vaccine in preventing severe gastroenteritis in young children according to socioeconomic status. Hum Vaccines Immunother 12: 2572-2579.
- Tharmaphornpilas P, Jiamsiri S, Boonchaiya S, Rochanathimoke O, Thinyounyong W, et al. (2017) Evaluating the first introduction of rotavirus vaccine in Thailand: moving from evidence to policy. Vaccine 35: 796-801.
- Fujii Y, Noguchi A, Miura S, Ishii H, Nakagomi T, et al. (2017) Effectiveness of rotavirus vaccines against hospitalisations in Japan. BMC pediatrics 17: 156.
- Desai SN, Esposito DB, Shapiro ED, Dennehy PH, Vázquez M (2010) Effectiveness of rotavirus vaccine in preventing hospitalization due to rotavirus gastroenteritis in young children in Connecticut, USA. Vaccine 28: 7501-7506.
- Marlow R, Ferreira M, Cordeiro E, Trotter C, Januário L, et al. (2015) Case control study of rotavirus vaccine effectiveness in Portugal during 6 years of private market use. Pediatr Infect Dis J 34: 509-512.
- Araki K, Hara M, Sakanishi Y, Shimanoe C, Nishida Y, et al. (2016) Estimating rotavirus vaccine effectiveness in Japan using a screening method. Hum Vaccines Immunother 12: 1244-1249.
- Yeung KHT, Tate JE, Chan CC, Chan MC, Chan PK, et al. (2016) Rotavirus vaccine effectiveness in Hong Kong children. Vaccine 34: 4935-4942.
- Patel M, Pedreira C, De Oliveira LH, Tate J, Orozco M, et al. (2009) Association between pentavalent rotavirus vaccine and severe rotavirus diarrhea among children in Nicaragua. JAMA 301: 2243-2251.
- Staat MA, Payne DC, Donauer S, Weinberg GA, Edwards KM, et al. (2011) Effectiveness of pentavalent rotavirus vaccine against severe disease. Pediatrics 128.
- Leshem E, Givon-lavi N, Tate JE, Greenberg D, Parashar UD, et al. (2016) Real-world effectiveness of pentavalent rotavirus vaccine among Bedouin and Jewish children in southern Israel. Clin Infect Dis 62: 155-160.
- Boom JA, Tate JE, Sahni LC, Rench MA, Hull JJ, et al. (2010) Effectiveness of pentavalent rotavirus vaccine in a large urban population in the United States. Pediatrics 125: e199-e207.
- Wang FT, Mast TC, Glass RJ, Loughlin J, Seeger JD (2010) Effectiveness of the Pentavalent Rotavirus Vaccine in Preventing Gastroenteritis in the United States. Pediatrics 125: e208-e213.
- Field EJ, Vally H, Grimwood K, Lambert SB (2010) Pentavalent Rotavirus Vaccine and Prevention of Gastroenteritis Hospitalizations in Australia. Pediatrics 126: e506-e512.
- Mast TC, Khawaja S, Espinoza F, Paniagua M, Del Carmen LP, et al. (2011) Case-control study of the effectiveness of vaccination with pentavalent rotavirus vaccine in Nicaragua. Pediatr Infect Dis J 30: e209-e215.
- Wang FT, Mast TC, Glass RJ, Loughlin J, Seeger JD (2012) Effectiveness of an Incomplete RotaTeq® (RV5) Vaccination Regimen in Preventing Rotavirus Gastroenteritis in the United States. Pediatr Infect Dis J 32: 1.
- Cardellino A, Khawaja S, Sánchez Cruz E, Mast TC (2013) Effectiveness of vaccination with the pentavalent rotavirus vaccine in Nicaragua as determined using the screening method. Hum Vaccin Immunother 9: 1449-1453.
- Yang T-A, Hou JY, Huang YC, Chen CJ (2017) Genetic Susceptibility to Rotavirus Gastroenteritis and Vaccine Effectiveness in Taiwanese Children. Sci Rep 7: 6412.
- Gastañaduy PA, Contreras-Roldán I, Bernart C, López B, Benoit SR, et al. (2016) Effectiveness of monovalent and pentavalent rotavirus vaccines in Guatemala. Clin Infect Dis 62: S121-S126.
- Yang TA, Hou JY, Huang YC, Chen CJ (2017) Genetic Susceptibility to Rotavirus Gastroenteritis and Vaccine Effectiveness in Taiwanese Children. Sci Rep 7: 6412.
- Sahakyan G, Grigoryan S, Wasley A, Mosina L, Sargsyan S, et al. (2016) Impact and effectiveness of monovalent rotavirus vaccine in Armenian children. Clin Infect Dis 62: S147-S154.
- Pérez-Vilar S, Díez-Domingo J, López-Lacort M, Martínez-Úbeda S, Martinez-Beneito MA, et al. (2015) Effectiveness of rotavirus vaccines, licensed but not funded, against rotavirus hospitalizations in the Valencia Region, Spain. BMC Infect Dis 15: 92.
- Sahakyan G, Grigoryan S, Wasley A, Mosina L, Sargsyan S, et al. (2016) Impact and effectiveness of monovalent rotavirus vaccine in Armenian children. Clin Infect Dis 62: S147-S154.
- Araujo EC, Clemens SAC, Oliveira CS, Justino MCA, Rubio P, et al. (2007) Safety, immunogenicity, and protective efficacy of two doses of RIX4414 live attenuated human rotavirus vaccine in healthy Brazilian infants. Pediatrics 83: 217-224.
- Gastañaduy PA, Steenhoff AP, Mokomane M, Esona MD, Bowen MD, et al. (2016) Effectiveness of monovalent rotavirus vaccine after programmatic implementation in Botswana: a multisite prospective case-control study. Clin Infect Dis 62: S161-S167.
- Tate JE, Ngabo F, Donnen P, Gatera M, Uwimana J, et al. (2016) Effectiveness of pentavalent rotavirus vaccine under conditions of routine use in Rwanda. Clin Infect Dis 62: S208-S212.
- Gheorghita S, Birca L, Donos A, Wasley A, Birca I, et al. (2016) Impact of rotavirus vaccine introduction and vaccine effectiveness in the Republic of Moldova. Clin Infect Dis 62: S140-S146.
- Nordgren J, Sharma S, Bucardo F, Nasir W, GünaydÃÆââ¬Å¾Ãâñn G, et al. (2014) Both Lewis and secretor status mediate susceptibility to rotavirus infections in a rotavirus genotype–dependent manner. Clin Infect Dis 59: 1567-1573.
- Moon SS, Groome MJ, Velasquez DE, Parashar UD, Jones S, et al. (2015) Prevaccination rotavirus serum IgG and IgA are associated with lower immunogenicity of live, oral human rotavirus vaccine in South African infants. Clin Infect Dis 62: 157-165.
- Moon SS, Tate JE, Ray P, Dennehy PH, Archary D, et al. (2013) Differential profiles and inhibitory effect on rotavirus vaccines of nonantibody components in breast milk from mothers in developing and developed countries. Pediatr Infect Dis J 32: 863.
- Moon SS, Wang Y, Shane AL, Nguyen T, Ray P, et al. (2010) Inhibitory effect of breast milk on infectivity of live oral rotavirus vaccines. Pediatr Infect Dis J 29: 919.
- Hoest C, Seidman JC, Pan W, Ambikapathi R, Kang G (2014) Evaluating associations between vaccine response and malnutrition, gut function, and enteric infections in the MAL-ED cohort study: methods and challenges. Clin Infect Dis 59: S273-S279.
- Prendergast AJ (2015) Malnutrition and vaccination in developing countries. Philos Trans R Soc Lond B Biol Sci 370: 20140141
- Wang H, Moon S, Wang Y, Jiang B (2012) Multiple virus infection alters rotavirus replication and expression of cytokines and Toll-like receptors in intestinal epithelial cells. Virus Res 167: 48-55.
- Filteau S (2009) The HIV-exposed, uninfected African child. Trop Med Int Health 14: 276-287.
- Chilengi R, Simuyandi M, Beach L, Mwila K, Becker-Dreps S, et al. (2016) Association of maternal immunity with rotavirus vaccine immunogenicity in Zambian infants. PloS one 11: e0150100.
- Mwila-Kazimbaya K, Garcia MP, Bosomprah S, Laban NM, Chisenga CC, et al. (2017) Effect of innate antiviral glycoproteins in breast milk on seroconversion to rotavirus vaccine (Rotarix) in children in Lusaka, Zambia. PloS one 12: e0189351.
- Mwila K, Chilengi R, Simuyandi M, Permar SR, Becker-Dreps S (2017) Contribution of Maternal Immunity to Decreased Rotavirus Vaccine Performance in Low- and Middle-Income Countries. Clin Vaccine Immunol 24: e00405-16.
- Mwape I, Bosomprah S, Mwaba J, Mwila-Kazimbaya K, Laban NM, et al. (2017) Immunogenicity of rotavirus vaccine (RotarixTM) in infants with environmental enteric dysfunction. PloS one 12: e0187761.
- Velasquez DE, Parashar UD, Jiang B (2014) Strain diversity plays no major role in the varying efficacy of rotavirus vaccines: An overview. Infect Genet Evol 28: 561-571.
- Leshem E, Lopman B, Glass R, Gentsch J, Bányai K, et al. (2014) Distribution of rotavirus strains and strain-specific effectiveness of the rotavirus vaccine after its introduction: a systematic review and meta-analysis. Lancet Infect Dis 14: 847-856.1.
- Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten M, et al. (2010) Effect of human rotavirus vaccine on severe diarrhoea in African infants. New Engl J Med 362: 289-298.
- Patel MM, Glass R, Desai R, Tate JE, Parashar UD (2012) Fulfilling the promise of rotavirus vaccines: how far have we come since licensure? Lancet Infect Dis 12: 561-570.
- Gurgel RQ, Cuevas LE, Vieira SC, Barros VC, Fontes PB, et al. (2007) Predominance of rotavirus P [4] G2 in a vaccinated population, Brazil. Emerg Infect Dis 13: 1571.
- Bernstein DI (2006) Live Attenuated Human Rotavirus Vaccine, RotarixTM. Semin Pediatr Infect Dis 17: 188-194.
- Rahman M, Sultana R, Ahmed G, Nahar S, Hassan ZM, et al. (2007) Prevalence of G2P [4] and G12P [6] rotavirus, Bangladesh. Emerg Infect Dis 13: 18.
- Groome MJ, Koen A, Fix A, Page N, Jose L, et al. (2017) Safety and immunogenicity of a parenteral P2-VP8-P [8] subunit rotavirus vaccine in toddlers and infants in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 17: 843-853.
- Bines JE, Danchin M, Jackson P, Handley A, Watts E, et al. (2015) Safety and immunogenicity of RV3-BB human neonatal rotavirus vaccine administered at birth or in infancy: a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 15: 1389-1397.
- Armah G E, Kapikian AZ, Vesikari T, Cunliffe N, Jacobson RM, et al. (2013) Efficacy, immunogenicity, and safety of two doses of a tetravalent rotavirus vaccine RRV-TV in Ghana with the first dose administered during the neonatal period. J infect Dis 208: 423-431.
- Burnett E, Lopman BA, Parashar UD (2017) Potential for a booster dose of rotavirus vaccine to further reduce diarrhoea mortality. Vaccine 35: 7198-7203.
- WHO | Rotavirus (RotaC) immunization coverage. WHO. 2017.
- Vogel F, Powell M (1995) A Summary Compendium of Vaccine Adjuvants and Excipients, Vaccine Design: The Subunit and Adjuvant Approach. Vol (Vogel F, Newman MJ, eds.). New York: Plenum Publishing.
- Demirjian A, Levy O (2009) Safety and efficacy of neonatal vaccination. Eur J Immunol 39: 36-46.
- Schneerson R, Fattom A, Szu SC, Bryla D, Ulrich JT, et al. (1991) Evaluation of monophosphoryl lipid A (MPL) as an adjuvant. Enhancement of the serum antibody response in mice to polysaccharide-protein conjugates by concurrent injection with MPL. J Immunol 147: 2136-2140.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences