Clinical Profile of Novel H1N1 Influenza in Children at a Tertiary Care Centre: Pune
Krishna Chaitanya, Archana Addanki, Nisha Deshpande and Rajendra Karambelkar
DOI10.21767/2573-0282.100058
Krishna Chaitanya*, Archana Addanki, Nisha Deshpande and Rajendra Karambelkar
Department of Pediatrics, Aditya Birla Memorial Hospital, Pune, Maharashtra, India
- *Corresponding Author:
- Chaitanya K
Department of Pediatrics, Aditya Birla Memorial Hospital
Pune, Maharashtra, India
E-mail: dkrishnac7@gmail.com
Received date: December 01, 2017; Accepted date: January 03, 2018; Published date: January 07, 2018
Citation: Chaitanya K, Addanki A, Deshpande N, Karambelkar N (2018) Clinical Profile of Novel H1 N1 Influenza in Children at a Tertiary Care Centre:Pune. Pediatric Infect Dis Vol 3:2. doi: 10.4172/2573-0282.100058
Copyright: © 2018 Chaitanya K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: With worldwide presence, Novel influenza virus (H1N1) carries great potential for causing a pandemic,there are limited studies about clinical profile of H1N1 in paediatric age group in India hence the present study was undertaken at Aditya Birla Memorial Hospital in Pune.
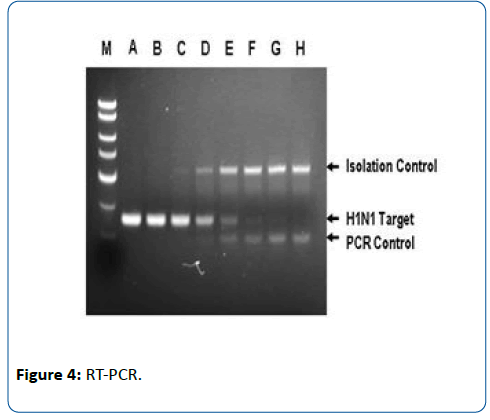
Methods: This was a prospective observational study, conducted during the time period from 01/03/2015 to 31/05/2016. Patients with influenza-like illness (Category B and C) aged 1 month to 16 yrs were included. Epidemiological, clinical and laboratory data was collected. For each patient one nasal and one throat swab was collected and sent to laboratory for RT-PCR (Real Time Polymerase Chain Reaction). All cases were treated with oral Oseltamivir according to MOHFW (Ministry Of Health and Family Welfare) guidelines. Data was analyzed for patients with positive RT-PCR.
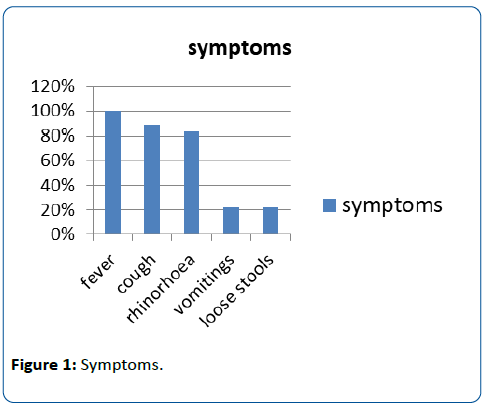
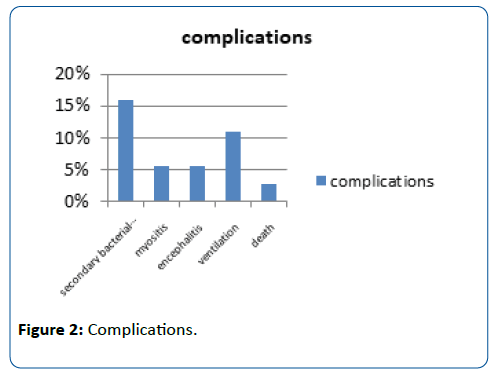
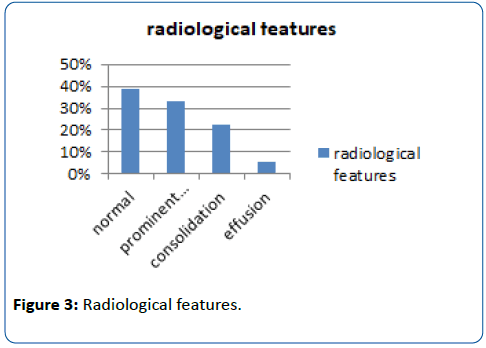
Results: Total 148 patients were enrolled in the study. H1N1 Influenza infection was confirmed on RT-PCR in 24.32% (n=36) patients. Majority of H1N1 infected patients (66.66%; n=24) were between 1-6 yrs and 22.22% were more than 6 yrs with mean age of 5.2 yrs. Male: Female ratio was 1.57:1. History of positive contact was obtained in 22.22% (n=8). In spite of earlier vaccination for H1N1 22.22% (n=8) suffered from infection.Common symptoms were fever (100%), cough (88.88%), rhinorrhoea (83.33%), vomitings (22.22%) and loose stools (22.22%). Normal chest X-ray was found in 38.88% of them, patchy consolidation in 33.33%, prominent broncho-vascular markings in 22.22% and pleural effusion in 5.55%. Fever lasted for more than 7 days in 33.33%. Secondary bacterial infection was found in 16.66% and 5.55% had evidence of myositis. 5.55% had evidence of encephalitis. Mechanical ventilation was required in 11% during the course of treatment. Mean duration of hospitalization was 4.1 days. One deaths occurred.
Keywords
H1N1 Influenza; Orthomyxoviridae; Influenza viruses; Pandemic Influenza
Introduction
After the detection of index case from India, amid a lot of panic, detection of Novel H1N1 infection was given focus. Pune was the epicenter of this pandemic and first death was also reported from Pune. The strain of Virus was result of triple reassortment containing genes from Avian, Human, Swine Influenza viruses [1]. The Gene segment Pb2 and PA from Duck influenza virus passed to Swine influenza virus in 1968. PB gene segment from duck transferred to human virus as well to swine influenza virus [1]. The Pandemic Influenza virus has the expression of all three genes [1]. Influenza virus which is an enveloped RNA virus of the Orthomyxoviridae family is having a potential for genetic variation dependant on the following characteristics.
1) The segmented genome, with eight RNA segments that are genetically independent of each other
2) A high rate of mutation, in the surface heamgglutinins (H) and neuraminidase (N) proteins. These properties along with the ability of the virus to spread infection in a wide host range of humans, domestic animals and birds makes it a potential pandemic agent [2].
The largest proportion of genes in this novel virus comes from swine influenza viruses (30.6%) from North American swine influenza strains, (17.5%) from Eurasian swine influenza strains), followed by North American avian influenza strains (34.4%) and human influenza strains (17.5%) [2]. It is unique genetic combination of influenza virus segments that was not seen before. Human being do not have the innate immunity to this virus and younger age group especially children are vulnerable to attack [1-3].
So the study was undertaken in Aditya Birla Memorial Hospital in Pune, among pediatric patients admitted, screened and treated for H1N1 flu illness to know about the demographic characteristics and clinical presentation features of this novel virus infection.
Material and Method
This was a prospective observational study, conducted during the time period from 01/03/2015 to 31/05/2016. Patients with symptoms of influenza-like illness (Category B and C) aged 1 month to 16 yrs were included in the study. Epidemiological, clinical and laboratory data was collected. For each patient one nasal and one throat swab was collected and sent to laboratory for analysis using indigenous RT-PCR (Real Time Polymerase Chain Reaction) kits which are based on Indian technology which used Taqman probes for influenza A. All cases were treated with oral Oseltamivir according to MOHFW (Ministry Of Health and Family Welfare) guidelines. Data was analyzed for patients with positive RT-PCR.
Influenza like illness was defined as Fever (Temperature of 1000 F/37.8°C) or greater, Sore throat, in the absence of known cause other than influenza [4-7].
Case definitions used in the study were: [8]
Suspected case: A person with acute febrile respiratory illness (reported or documented fever, and one of the following: cough, sore throat, shortness of breath, difficulty in breathing or chest pains) with onset:
• within 7 days of close contact with a person who is aprobable or confirmed case of the new influenza (H1N1) virus infection, or
• within7 days of travel to a community internationally where there has been one or more confirmed Pandemic influenza (H1N1) cases, or
• resides in a community where there are one or more confirmed new influenza cases.
Confirmed case: A Confirmed case of Pandemic Influenza (H1N1) virus infection is defined as an individual with laboratory confirmed new influenza (H1N1) virus infection by positive real time reverse transcriptase polymerase chain reaction (RT-PCR).
As per orders of central government, MOHFW has categorized patients of suspected swine flu into three (A, B and C) categories which are as follows:
Category A: Patients with mild fever, cough and sore throat, body ache, headache, nausea and diarrhoea are put in Category A and are monitored for 24-48 h. These patients are advised to stay at home and not mingle with the others. These patients do not need testing for H1N1 and no treatment with Oseltamivir.
Category B: Those patients who have all the symptoms mentioned in Category A, but have high-grade fever and are in the high-risk category; they need treatment with Oseltamivir and are advised to remain confined at home. “High-risk category includes children with mild illness, pregnant women, persons over 65, patients with lung, liver, heart, kidney, blood or neurological diseases or have been on long-term cortisone therapy.”
Category C: Those patients who have breathlessness, chest pain, drowsiness, fall in blood pressure, sputum mixed with blood, bluish discolouration of nails and who need to be immediately started on the medicine and hospitalised. This category also includes children with influenza-like illness, high and persistent fever, inability to feed, convulsions and difficulty in breathing.
Epidemiological, clinical and laboratory data was collected for each patient. For each patient one nasal and one throat swab was collected and sent to laboratory for RT-PCR (Real Time Polymerase Chain Reaction). All cases were treated with oral Oseltamivir according to MOHFW (Ministry Of Health and Family Welfare) guidelines. Data was analyzed for patients with positive RT-PCR.
Result
Total 148 patients were enrolled in the study. H1N1 Influenza infection was confirmed on RT-PCR in 24.32% (n=36) patients. Majority of H1N1 infected patients (66.66%; n=24) were between 1-6 yrs and 22.22% were more than 6 yrs with mean age of 5.2 yrs. Male: Female ratio was 1.57:1. History of positive contact was obtained in 22.22% (n=8). Prior vaccination history for H1N1 was present for 22.22% (n=8). Common symptoms were fever (100%), cough (88.88%), rhinorrhoea (83.33%), vomitings (22.22%) and loose stools (22.22%). The signs observed were congested posterior pharynx (38.88%), bilateral crepitations in chest (27.77%), bronchial breathing (22.22%), congested tonsils (16.66%) and tachypnoea (11.11%). Normal chest X-ray was found in 38.88% of them, patchy consolidation in 33.33%, prominent broncho-vascular markings in 22.22% and pleural effusion in 5.55%. TLC (total leukocyte count) was <5000/cmm in 25% and 16.66% had TLC>14000/cmm with lymphocytic predominance in 66.66% of them. Fever lasted for more than 7 days in 33.33%. Secondary bacterial infection was found in 16.66% and 5.55% had evidence of myositis. 5.55% had evidence of encephalitis. Mechanical ventilation was required in 11% during the course of treatment. Mean duration of hospitalization was 4.1 days. Case fatality rate in our study was 2.8% (n=1) (Figures 1-4).
Discussion
In India, the first case reported was from Pune and the pandemic has spread quite rapidly with more than 2,000 confirmed cases affected both sexes equally and lead to 25 deaths in the initial phase of the epidemic itself. Children and Young adults were commonly affected and nearly 40% of those affected were children less than 14 yrs [9]. H1N1 is transmitted by droplets or fomites and the incubation period is 2 to 7 days. Most of them present with mild symptoms in the form of fever, cough, sore throat, headache, joint pain and myalgia [10]. Patients at risk of developing severe disease include age less than 5 yrs, chronic systemic illness, on steroids or immunosuppressive therapy. Children younger than 2 yrs have the highest complication rates. The mean age of affected children in our study was 5.2 yrs which was similar to the previous studies [11,12] probably because this group of children have a transition of exposure of environment from home to schools.
A feature seen more frequently with swine flu origin influenza is gastrointestinal symptoms with, almost one fourth of them presenting with vomiting and diarrhea which is similar to those of previous studies [11,12]. Unusual symptoms reported are conjunctivitis, parotitis, and hemophagocytic syndrome [13] were not observed in our study probably due to smaller sample size and shorter duration. Literature showed that less than 10% of Children present with severe manifestations in the form of severe pneumonia and respiratory failure and need prolonged hospitalization [14], but higher percentage of patients in this study (33.33%) showed radiological evidence of pneumonia. The case fatality is reported to less than 1% in the previous studies [11,12], however it was 2.8% in our study probably due to higher incidence of pneumonia like complications or due to small sample size.
Significant finding of this study is that good percentage (22.22%) of people who were vaccinated previously were also got affected but the severity of illness is less among them as compared to those not vaccinated.
Conclusion
H1N1 influenza most commonly affects children 4-6 yrs of age followed by adolescents. Slight male preponderance was found. Clinical features and routine laboratory investigations in children with Novel H1N1 influenza were similar to other influenza like infections. Features like severe vomitings, diarrhoea, respiratory distress, wheezing, and infiltrates/consolidation on chest radiograph were common indications for admission. Vaccination does not completely prevent from getting infected but definitely decreases the severity of illness. Most children with flu symptoms do not need a test for pandemic H1N1/09 especially since the test results do not affect the recommended course of treatment.
References
- Prasad K (2009) Influenza activities in the past & last 6 months. Influenza Foundation of India. https://www.influenzafoundationofindia.org/burden-of-influenza.
- Bhushan B, Nagaraja C (2009) Novel H1N1 influenza epidemic: Lessons from A Tertiary Centre In Bangalore. BMC Pulm Med 12: 35-39.
- Chandrashekar T (2012) H1N1 infected patients in ICU & their clinical outcome. N Am J Med Sci 4: 394–398.
- Kaore M, Kaore S, Sharma P (2009) Laboratory diagnosis of Novel H1N1 Virus. JK Science 11: 15-18.
- Siddhivinayak H, Chadha M, Lele P, Lafond KE, Deoshatwar A, et al. (2012) Performance of case definitions used for influenza surveillance among hospitalized patients in a rural area of India. Bull World Health Organ 90: 804-812.
- Tambe MP, Parande M, Jamkar AV, Pardesi RR, Baliwant K, et al. (2012) An epidemiological study of confirmed H1N1 admitted cases in an infectious disease hospital, Pune. J Krishna Inst Med Sci Univ 1: 81-90.
- Chadha M, Hirve S, Dawood F, Lele P, Deoshatwar A, et al. (2013) Burden of Seasonal and Pandemic Influenza-Associated Hospitalization during and after 2009 A(H1N1) pdm09 Pandemic in a Rural Community in India. PLoS ONE 8: e55918.
- Pandemic Influenza (H1N1) (2009) CD Alert 13: 1-8.
- Ministry of Health and Family Welfare. Government of India. Influenza A (H1N1). https://mohfw.nic.in.
- Myers K, Olsen C, Gray G (2007) Cases of Swine Influenza in Humans: A Review of the Literature. Clin Infect Dis 44: 1084-1088.
- Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, Hernandez M, Quiñones-Falconi F, et al. (2009) Pneumonia and Respiratory Failure from Swine-Origin Influenza A (H1N1) in Mexico. N Engl J Med 361: 680-689.
- https://www.cdc.gov/h1n1flu/recommendations.html.
- Bastien N, Bowness D, Burton L (2009) Parotitis in a child infected with triple reassortment influenza Avirus in Canada in 2007. J Clin Microbiol 47: 1896–1898.
- https://www.who.int/csr/resources/publications/swineflu/clinical_management/en/index.html.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences