Advancements in the Therapy of Ebola Virus Disease
Hiewa Othman Dyary, Heshu Sulaiman Rahman, Hemn Hassan Othman, Rasedee Abdullah, Max Stanley Chartrand
DOI10.21767/2573-0282.100022
Hiewa Othman Dyary1*, Heshu Sulaiman Rahman1,2,3, Hemn Hassan Othman1,2, Rasedee Abdullah3, Max Stanley Chartrand4
1College of Veterinary Medicine, University of Sulaimani, Kurdistan Region, Northern Iraq
2College of Health Sciences, Department of Medical Laboratory Sciences, Komar University of Science and Technology, Sulaimany City, Kurdistan Region, Northern Iraq
3Faculty of Veterinary Medicine, University Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia
4Digi Care Behavioral Research, Casa Grande, Arizona, USA
- *Corresponding Author:
- Hiewa Othman Dyary
College of Veterinary Medicine
University of Sulaimani, Kurdistan Region
Northern Iraq
E-mail: dyary.othman@univsul.edu.iq
Received date: July 12, 2016; Accepted date: August 11, 2016; Published date: August 18, 2016
Citation: Dyary HO, Rahman HS, Othman HH, Abdullah R, Chartrand MS (2016) Advancements in the Therapy of Ebola Virus Disease. Pediatric Infect Dis 1:22. doi: 10.21767/2573-0282.100022
Abstract
Ebola virus disease is an infection of human and nonhuman primates with fatality rates of up to 90%. Since 2014, the largest outbreak of Ebola virus in recorded history spread into several adjacent West African nations. The infection especially propagated into highly populated areas and where there was inadequate healthcare infrastructure and public sanitation. Such deficiencies permitted the widespread of the virus and caused more than 10,000 casualties to-date. Currently, no specific therapy is available and treatment of Ebola patients depends mainly on supportive care and symptomatic treatment. A worldwide effort has been made to develop new therapeutic strategies, several of which were potential vaccines where promising results were demonstrated in non-human primates. High throughput screening of FDA-approved drugs has revealed several compounds with potential anti-Ebola activity in vitro, which raises the idea of reconsidering the previously approved drugs as possible candidates against the virus. This article reviews the current drug candidates and prospects towards the development of potential EVD therapy.
Keywords
Ebola virus; Therapy; Review article
Introduction
Viral hemorrhagic fevers are several diseases caused by a group of enveloped RNA-virus families that cause febrile disease with haemorrhagic symptoms. These include the arena, bunya, filo and flaviviruses [1].
Ebola Virus Disease (EVD) is a disease caused by infection with one of the five Ebola virus species of the family Filoviridae [2]. Four species, namely Sudan ebolavirus, Zaire ebolavirus, Bundibugyo ebolavirus and Cote d’Ivoire ebolavirus are capable of human infection with fatal outcomes. Pigs and nonhuman primates (NHPs) are susceptible to infection with Reston ebola virus while humans are only infected asymptomatically [3].
The first outbreak of EVD was reported in 1967 in Zaire (The Democratic Republic of the Congo) resulting in 318 cases and 88% death rate [4]. The 2014 EVD outbreak, which was caused by Zaire ebola virus and spread in the West African countries Liberia, Sierra Leone and Guinea, was the largest Ebola outbreak with more than 28500 reported human cases and mortality rates reaching 39.7% [5].
The disease course of EVD is rapid. After about one week incubation period, victims rapidly develop a high fever, diarrhea, vomiting, respiratory disorders, haemorrhaging and death ensues within a few days [6].
Transmission of EVD occurs through acquiring infection from the handling of wild animals such as fruit bats, antelopes, chimpanzees, gorillas, monkeys and porcupines [7]. The virus spreads from an infected to a healthy person via contact with the infected person’s skin, body fluids or blood [8,9].
Currently, there are no approved drugs for the therapy of EVD, and management of the disease is largely dependent on the maintenance of intravascular fluid volume of the patients [10] and palliative care, even though some tested compounds exhibit promising antiviral activities against the ebolavirus. This review summarizes the current advancements in the therapy of EVD in the effort towards the development of therapeutic agents against Ebola virus.
Treatment of Ebola Virus Infections
The principal strategies currently followed in the treatment of EVD are confined to the symptomatic and supportive care of patients, by maintaining fluid, electrolytes and acid-base balance of blood, and treatment of secondary infections [11]. There are no approved therapies specific for EVD at present [12].
Supportive Care of EVD Patients
Ebola virus disease is an acute febrile disease characterized by muscle pain, weakness, diarrhea and vomiting [13]. Supportive care of EVD patients aims at restoring the balance of the body’s fluid and electrolytes that are otherwise lost through the gastrointestinal tract [10,14]. Patients are given oral or intravenous fluid compensate for the amount lost from severe diarrhea. The restoration of potassium, magnesium, and calcium ion levels in the blood is also deemed necessary [15].
Management of the disease symptoms is required to reduce the severity of these symptoms. Nausea and vomiting are treated through the administration of antiemetics, such as metoclopramide and ondansetron [12]. Diarrhea is treated by using antidiarrheal agents such as loperamide [16]. Pain is managed by using acetaminophen or one of the opioid analgesic agents [12]. Antibacterial agents are sometimes administered to the infected patients as they are thought to prevent translocation of bacteria from the gastrointestinal tract [17,18]. In some patients, respiratory failure may develop. Respiratory support is required in these patients [19].
Therapeutic Approach to EVD
Currently, no specific compounds have been approved for the therapy of EVD [20,21]. The management of EVD depends on replacement of the patient’s fluid and electrolytes that are lost through diarrhea, as well as reducing the severity of the disease symptoms such as diarrhea and muscle pain.
Current therapeutic approach to EVD has been focused on the implementation of different strategies as the use of convalescent plasma and monoclonal antibodies, and in testing compounds that inhibit the entry and fusion of Ebola virus into the target cells or possess viral polymerase enzyme inhibitory activity. The 2014 Ebola outbreak has increased the pace towards the development of an effective Ebola vaccine as well. The following sections focus on the current efforts towards the development of Ebola therapy and promising vaccine platforms that undergo clinical trials.
Convalescent Plasma and Monoclonal Antibodies
The use of convalescent plasma from patients who survived infection with EVD provides immediate protection to infected patients. The use of immunoglobulin isolated from equine hyper-immune serum to protect hamadryas baboons (Papio hamadryas) against EVD was attempted by Borisevich et al. in 1995. The immunoglobulin conferred up to 100% protection against EVD when tested in hamadryas baboons [22]. In another study, immune plasma high in antibody titer against EVD was taken from immunized sheep and goats and tested on experimentally infected guinea pigs. The transfusion provided prophylaxis within 48 hours after infection [23]. The World Health Organization (WHO) reckons the use of convalescent whole blood or plasma from survivors as a possible therapy of EVD [24]. Transfusion therapy is considered an inexpensive method to save the lives of infected patients and the use of convalescent plasma is predicted to result in lower mortality rates than the use of convalescent whole blood [25].
Transfusions of high concentrations of neutralizing antibodies can confer protection against different viral infections [26]. The use of monoclonal antibodies in the inhibition of EVD has been attempted in the past. It was shown that a human neutralizing monoclonal antibody, KZ52, confers protection in guinea pigs against experimental EVD infection [27]. However, KZ52 failed to prevent experimental EVD infection in macaques. It was assumed that the Ebola virus uses a propagation or mutation mechanism that was insensitive to high concentrations of neutralizing antibody. The success of such vaccinations may be dependent upon the cooperation of other antibody and cellular immunity [28].
Recently, a new monoclonal antibody cocktail, called ZMapp, which is a combination of monoclonal antibodies, was prepared from components of MB-003 and ZMab antibody cocktails and was tested on rhesus macaques. ZMapp was capable of providing 100% protection against experimental infection in the macaques when the cocktail was administered intravenously up to five days post intramuscular injection of a lethal dose of the virus [29].
Polymerase Inhibitors
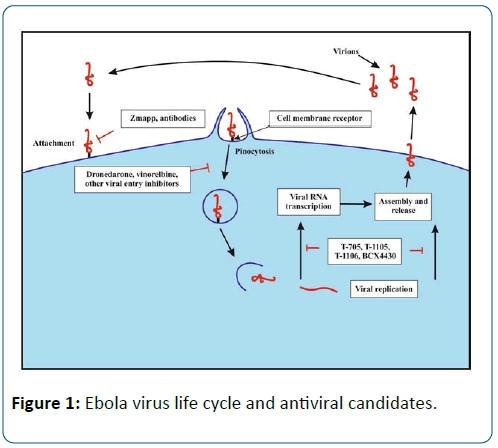
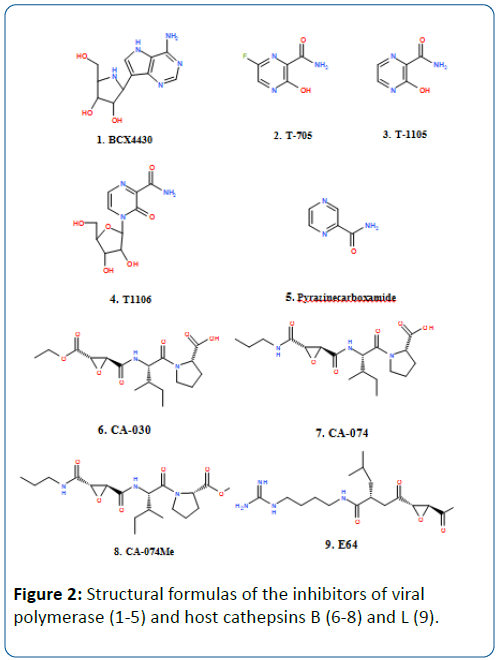
Several viral polymerase inhibitors have been reported to exhibit efficacy against Ebola virus. BCX4430 is a synthetic analog of adenosine (Figure 1) that inhibits the RNA polymerase resulting in the termination of the RNA chain. This compound was earlier reported to protect against Ebola virus and Marburg virus infection when administered intramuscularly in mice models. Cynomolgus macaques were also protected against Marburg virus after 48 hours of virus infection [30].
Three derivatives of pyrazine carboxamide, T-705 (favipiravir), T-1105 and T-1106, have been reported to have antiviral activity against different RNA virus infections in laboratory animals, such as arenaviruses, bunyaviruses, influenza virus, West Nile virus, yellow fever virus, and footand- mouth disease virus [31]. Of these compounds, T-705 is currently undergoing phase III clinical trials for use against influenza infection. The compound has shown inhibitory activity against Ebola virus in cell culture and has also been reported to confer 100% protection against Ebola virus infection to immune-deficient mice after a daily administration for two weeks [32]. In December 2014, a clinical trial of T-705 against EVD was started in Guinea to evaluate the efficacy of the compound against Ebola virus infection. Even though there were concerns about teratogenic and embryotoxic effects of the compound, the Japanese Ministry of Health and labor approved the use of this compound with strict regulations [33]. T-705 is converted to the active ribofuranosyl triphosphate derivative form by the host enzymes, which in turn inhibits the viral RNA-dependent RNA polymerase without inhibition of mammalian DNA or RNA [31].
Three derivatives of pyrazine carboxamide, T-705 (favipiravir), T-1105 and T-1106, have been reported to have antiviral activity against different RNA virus infections in laboratory animals, such as arenaviruses, bunyaviruses, influenza virus, West Nile virus, yellow fever virus, and footand- mouth disease virus [31]. Of these compounds, T-705 is currently undergoing phase III clinical trials for use against influenza infection. The compound has shown inhibitory activity against Ebola virus in cell culture and has also been reported to confer 100% protection against Ebola virus infection to immune-deficient mice after a daily administration for two weeks [32]. In December 2014, a clinical trial of T-705 against EVD was started in Guinea to evaluate the efficacy of the compound against Ebola virus infection. Even though there were concerns about teratogenic and embryotoxic effects of the compound, the Japanese Ministry of Health and labor approved the use of this compound with strict regulations [33]. T-705 is converted to the active ribofuranosyl triphosphate derivative form by the host enzymes, which in turn inhibits the viral RNA-dependent RNA polymerase without inhibition of mammalian DNA or RNA [31].
Inhibitors of Virus Entry and Fusion
Fusion of the viral and host cell membranes comprises the first step in Ebola virus infection. Molecules inhibiting the viral fusion and entry into the target cells are potential candidates for the development of new therapeutics against Ebola virus. Recently, a high throughput in vitro assay was developed using a 1536-well plate to screen Food and Drug Administration (FDA)-approved drugs for molecules that inhibit the entry of Ebola virus-like particles [34]. The screening resulted in the identification of 53 compounds that exhibited an inhibitory effect on virus-like particles. The majority of these compounds were previously approved for use as anticancer (e.g., vinblastine, vinorelbine/navelbine and vincristine), anthelmintic (e.g., mebendazole and albendazole), antiarrhythmic (e.g., digoxin and Dronedarone) and antidepressant (e.g., Maprotiline and Clomipramine) agents. In another in vitro assay for repurposing FDA-approved drugs [35]. 1,012 compounds were screened for antiviral activity against Ebola and other viruses. Among the tested compounds, chloroquine (antimalarial), estradiol (estrogen), toremifene (selective modulator of estrogen receptor), diphenoxylate (anti-peristaltic) and dipivefrin (treatment of glaucoma) were reported to inhibit viral fusion events. The results of the foregoing assay demonstrate the advantages of re-purposing drugs that are already in use for the treatment of other ailments. However, further in vivo studies should be conducted to prove efficacy of these compounds against Ebola virus infection.
The entry of Ebola virus particles into the host cell is mediated by proteolysis of the virus glycoprotein via two protease enzymes of the host cell, cathepsin B and Cathepsin L, demonstrating the contributing action of these enzymes to infection [36]. Selective inhibitors of cathepsin B (e.g., CA-030, CA-074, CA-074Me) and cathepsin L (e.g., E-64c) could serve as potential candidates in the therapy of EVD [37,38]. However, Marzi et al. [39] demonstrated that these two enzymes weren’t necessary for virus entry and replication. The cleavage of Ebola virus glycoprotein appears to be mediated by a series of protease enzymes, which makes the development of specific drugs a difficult task.
Development of anti-Ebola Vaccines
No approved vaccine is currently available to protect against Ebola virus. A number of vaccines have been reported to provide protection against the infection in small laboratory animals and NHPs. Laboratory animal models such as guinea pigs and mice serve as suitable alternatives to clinical trials of vaccines in human beings [40]. A vesicular stomatitis virusbased vaccine expressing Ebola glycoprotein was tested using two laboratory animal models. In the first model, the vaccine was able to confer 100% protection to mice challenged with a lethal dose of mouse-adapted Ebola virus at nine months after vaccination. In the second model, guinea pigs were challenged with a lethal dose of Ebola virus at seven months postvaccination, where 83% of the animals were found to survive the infection [41].However, rodent animals are not susceptible to infection with natural Ebola virus and viral adaptation in these animals is required for infection [40]. Therefore, this vaccine may not actually be demonstrating efficacy as used in these studies.
Since NHPs are highly susceptible to Ebola virus infection with a high mortality rate that is similar to the susceptibility of humans to Ebola infection, they represent the best choice for evaluation of Ebola vaccine efficacy. A vaccine consisting of Ebola envelope glycoprotein in an adenoviral vector provided an extremely effective protection to cynomolgus macaques (Macaca fascicularis) when they were challenged with Ebola virus 28 days after vaccination [42]. The vector-based vaccines provide adequate protection to NHPs. In a phase I clinical trial,the envelope glycoprotein from Zaire and Sudan Ebola virus species was encoded in a recombinant adenovirus vaccine. The vaccine was tested in healthy adults (n=23) and was proven safe and provided cellular and humoral immune responses [43].
A chimpanzee adenovirus vector-derived vaccine encoding Ebola virus glycoprotein (ChAd3-ZEBOV-GP) was able to confer protective immunity against an acute lethal dose of Zaire Ebola virus (EBOV) challenge in macaques [44]. Another vaccine expressing individual filovirus glycoproteins on a recombinant vesicular stomatitis virus vector has demonstrated 100% protection against filovirus infection in NHPs. Cynomolgus macaques were immunized with the vaccine and subsequently challenged with Marburg virus after 28-35 days of vaccination. Viremia was not detected in any of the vaccinated animals after the challenge and no signs of clinical infection were reported in the challenged animals [45]. Both vaccines are currently undergoing Phase I and Phase II clinical trials [46].
The non-replicative vector-based vaccines conferred a high level of immunization when tested in NHPs with the hope of eventually developing a successful EVD vaccine. However, a large dose of the vaccine is required to confer immunization satisfactorily in NHPs, and this makes the manufacturing of the vaccine under Good Manufacture Practice (GMP) a difficult and costly task [47]. Moreover, these vaccines provide a low level of immunization when tested in humans, and it is not clear whether a higher dose of the vaccine will be effective in providing a desirable level of the immune response [43]. Moreover, the vaccines fail to induce significant levels of immunization in individuals who had previously been infected with the viral vector, such as adenovirus, due to the presence of pre-existing immunity [43].
Concluding Remarks
Various strategies that have been followed in therapeutic applications in cases of EVD, together with an increased pace of experimental trials following the 2014 Ebola outbreak, show promise in the development of an effective Ebola therapy in the very near future. Successful application with monoclonal antibodies cocktail ZMapp in protecting macaques against Ebola infection shows particular promise in this antibody cocktail in human clinical trials. RNA polymerase inhibitors, such as BCX4430 and T-705 (favipiravir) (Figure 2), which have shown efficacy against EVD in animal models, are also considered potential candidates for new drug applications against Ebola infection. And despite concerns about the teratogenic and embryotoxic effects of T-705, this compound has been approved by the Japanese Ministry of Health and Labor for therapy against EVD.
Furthermore, the development of vector-derived vaccines, such as vesicular stomatitis virus-based vaccine expressing Ebola glycoprotein, has shown ample protection against the virus challenge in NHPs. However, the vector-based vaccines conferred a small level of protection when human beings were immunized.
Perhaps the best approach towards the development of therapeutics against EVD is the repurposing of FDA-approved drugs for the treatment of Ebola infection. Several previously approved therapies have demonstrated inhibitory effects on the Ebola viral fusion and entry in vitro. However, screening of the large number of compounds approved by the FDA calls for the implementation of high-throughput techniques to test the compounds in a short period of time.
The recurring outbreaks of Ebola during 2014 call for urgency in developing new drugs and therapeutic strategies. Locales where humans live in close contact with wild animals that can serve as virus reservoirs make the control of EVD are particularly at risk and pose the most difficult task to protect from infection. Therefore, strategic utilization of the best currently available drugs and therapies is, in our opinion, the best approach for control and prevention of the Ebola infection in such populations until better strategies and vaccines can be developed.
References
- Sanchez A, Kiley MP, Holloway BP, Auperin DD (1993) Sequence analysis of the Ebola virus genome: organization, genetic elements, and comparison with the genome of Marburg virus. Virus Res 29: 215-240.
- Groseth A, Hoenen T, Eickmann M, Becker S (2011) "Filoviruses: Ebola, marburg and disease" John Wiley & Sons, Ltd.
- To KK, Chan JF, Tsang AK, Cheng VC, Yuen KY (2015) Ebola virus disease: a highly fatal infectious disease reemerging in West Africa. Microbes Infect 17: 84-97.
- [No authors listed] (1978) Ebola haemorrhagic fever in Zaire, 1976. See comment in PubMed Commons below Bull World Health Organ 56: 271-293.
- https://apps.who.int/ebola/ebola-situation-reports
- Pourrut X, Kumulungui B, Wittmann T, Moussavou G, Délicat A, et al. (2005) The natural history of Ebola virus in Africa. Microbes Infect 7: 1005-1014.
- Jarrett A (2015) "Ebola: A practice summary for nurse practitioners". J Nurse Pract 11: 16-26.
- Rewar S, Mirdha D (2014) Transmission of ebola virus disease: an overview. Ann Glob Health 80: 444-451.
- Shears P, O'Dempsey TJ (2015) Ebola virus disease in Africa: epidemiology and nosocomial transmission. J Hosp Infect 90: 1-9.
- Fowler RA, Fletcher T, Fischer WA, Lamontagne F, Jacob S, et al. "Caring for critically ill patients with ebola virus disease. Perspectives from west africa". Am J RespirCrit Care Med 190: 733-737.
- Tseng CP, Chan YJ (2015) Overview of Ebola virus disease in 2014. J Chin Med Assoc 78: 51-55.
- Wong K. K. and Uyeki T. M., "Clinical management of ebola virus disease patients". Curr Treat Options Infect Dis 7: 248-260.
- Bah EI, Lamah MC, Fletcher T, Jacob ST, Brett-Major DM, et al. "Clinical presentation of patients with ebola virus disease in conakry, guinea". N Engl J Med 372: 40-47.
- World Health Organization (2014) Clinical Management of Patients with Viral Haemorrhagic Fever: a Pocket Guide for the Front-Line Health Worker: Interim Emergency Guidance-Generic Draft for West African Adaptation.
- Wong KK, Perdue CL, Malia J, Kenney JL, Peng S, et al. (2015) "Supportive care of the first two ebola virus disease patients at the monrovia medical unit" Clin Infect Dis 61: e47-e51.
- Chertow DS, Uyeki TM, DuPont HL (2015) "Loperamide therapy for voluminous diarrhea in ebola virus disease". J Infect Dis 61: 609-614.
- O'Shea MK, Clay KA, Craig DG, Matthews SW, Kao RL, et al. (2015) "Diagnosis of febrile illnesses other than ebola virus disease at an ebola treatment unit in sierra leone". Clin Infect Dis 61: 795-798.
- Ansumana R, Jacobsen KH, Idris Mb, Bangura H, Boie-Jalloh M, et al. (2015) "Ebola in freetown area, sierra leone-a case study of 581 patients". N Engl J Med 372: 587-588.
- Wolf T, Kann G, Becker S, Stephan C, Brodt HR, et al. (2015) Severe Ebola virus disease with vascular leakage and multiorgan failure: treatment of a patient in intensive care. Lancet 385: 1428-1435.
- Yazdanpanah Y, Arribas JR, Malvy D (2015) Treatment of Ebola virus disease. Intensive Care Med 41: 115-117.
- Martínez-Romero C, García-Sastre A (2015) "Against the clock towards new ebola virus therapies". Virus research 209: 4-10.
- Borisevich I, Mikhailov V, Krasnianskii V, Gradoboev V, Lebedinskaia E, et al. (1994) "Development and study of the properties of immunoglobulin against ebola fever". VoprosyVirusologi 40: 270-273.
- Kudoyarova-Zubavichene NM, Sergeyev NN, Chepurnov AA, Netesov SV (1999) "Preparation and use of hyperimmune serum for prophylaxis and therapy of ebola virus infections". J Infect Dis 179: S218-S223.
- Gutfraind A. and Meyers L. A., "Evaluating large-scale blood transfusion therapy for the current ebola epidemic in liberia" Journal of Infectious Diseases. Vol. (211), No. (8), pp. 1262-1267. (2015).
- Hangartner L, Zinkernagel RM, Hengartner H (2006) "Antiviral antibody responses: The two extremes of a wide spectrum". Nat Rev Immunol 6: 231-243.
- Parren PW, Geisbert TW, Maruyama T, Jahrling PB, Burton DR (2002) "Pre-and postexposure prophylaxis of ebola virus infection in an animal model by passive transfer of a neutralizing human antibody". J Virol 76: 6408-6412.
- Oswald WB, Geisbert TW, Davis KJ, Geisbert JB, Sullivan NJ, et al. (2007) "Neutralizing antibody fails to impact the course of ebola virus infection in monkeys". PLoSPathog 3: e9.
- Qiu X, Wong G, Audet J, Bello A, Fernando L, et al. (2014) Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 514: 47-53.
- Warren TK, Wells J, Panchal RG, Stuthman KS, Garza NL, et al. "Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue bcx4430". Nature 508: 402-405.
- Furuta Y, Takahashi K, Shiraki K, Sakamoto K, Smee DF, et al. (2009) "T-705 (favipiravir) and related compounds: Novel broad-spectrum inhibitors of rna viral infections". Antiviral Re 82: 95-102.
- Smither SJ, Eastaugh LS, Steward JA, Nelson M, Lenk RP, et al. (2014) "Post-exposure efficacy of oral t-705 (favipiravir) against inhalational ebola virus infection in a mouse model". Antiviral Re 104: 153-155.
- Nagata T, Lefor AK, Hasegawa M, Ishii M (2015) "Favipiravir: A new medication for the ebola virus disease pandemic". Disaster Med Public Health Prep 9: 79-81.
- Kouznetsova J, Sun W, Martínez-Romero C, Tawa G, Shinn P, et al. (2014) "Identification of 53 compounds that block ebola virus-like particle entry via a repurposing screen of approved drugs" Emerg Microbes Infect 3: e84.
- Madrid PB, Chopra S, Manger ID, Gilfillan L, Keepers TR, et al. (2013) "A systematic screen of fda-approved drugs for inhibitors of biological threat agents". PloS One 8: e60579.
- Chandran K, Sullivan NJ, Felbor U, Whelan SP, Cunningham JM (2005) Endosomal proteolysis of the Ebola virus glycoprotein is necessary for infection. Science 308: 1643-1645.
- Murata M, Miyashita S, Yokoo C, Tamai M, Hanada K, et al. (1991) "Novel epoxysuccinyl peptides selective inhibitors of cathepsin b, in vitro". FEBS Letters 280: 307-310.
- Gnirb K, Kuhl A, Karsten C, Glowacka I, Bertram S, et al. (2012) "Cathepsins b and l activate ebola but not marburg virus glycoproteins for efficient entry into cell lines and macrophages independent of tmprss2 expression" Virology 424: 3-10.
- Marzi A, Reinheckel T, Feldmann H (2012) Cathepsin B & L are not required for ebola virus replication. PLoSNegl Trop Dis 6: e1923.
- Bray M, Davis K, Geisbert T, Schmaljohn C, Huggins J (1998) A mouse model for evaluation of prophylaxis and therapy of Ebola hemorrhagic fever. J Infect Dis 178: 651-661.
- Wong G, Audet J, Fernando L, Fausther-Bovendo H, Alimonti JB, et al. (2014) "Immunization with vesicular stomatitis virus vaccine expressing the ebola glycoprotein provides sustained long-term protection in rodents". Vaccine 32: 5722-5729.
- Sullivan NJ, Geisbert TW, Geisbert JB, Xu L, Yang ZY, et al. (2003) Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates. Nature 424: 681-684.
- Ledgerwood J, Costner P, Desai N, Holman L, Enama M, et al. (2010) "A replication defective recombinant ad5 vaccine expressing ebola virus gp is safe and immunogenic in healthy adults" Vaccine 29: 304-313.
- Stanley DA, Honko AN, Asiedu C, Trefry JC, Lau-Kilby AW, et al. (2014) "Chimpanzee adenovirus vaccine generates acute and durable protective immunity against ebolavirus challenge". Nat Med 20: 1126-1129.
- Mire CE, Geisbert JB, Agans KN, Satterfield BA, Versteeg KM, et al. (2014) "Durability of a vesicular stomatitis virus-based marburg virus vaccine in nonhuman primates". PloS One 9: e94355.
- Cooper CL, Bavari S (2015) A race for an Ebola vaccine: promises and obstacles. Trends Microbiol 23: 65-66.
- Ye L, Yang C (2015) Development of vaccines for prevention of Ebola virus infection. Microbes Infect 17: 98-108.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences