Bronchoscopic Abnormalities in Pediatric Chronic Cough: A Case-Control Study
Mikel Santiago-Burruchaga, Estibaliz Catediano-Sainz, Rafael Zalacain- Jorge and Carlos Vazquez- Cordero
DOI10.21767/2573-0282.100010
Mikel Santiago-Burruchaga1*, Estibaliz Catediano-Sainz1, Rafael Zalacain- Jorge 2 and Carlos Vazquez- Cordero1
1Paediatric Pulmonology Unit, Paediatric Department, Hospital Universitario de Cruces, Spain
2Pulmonology Department, Hospital Universitario de Cruces, Spain
- *Corresponding Author:
- Mikel Santiago-Burruchaga
Paediatric Pulmonology Unit
Paediatric Department
Hospital Universitario de Cruces, Spain
E-mail: mikelsanti@euskalnet.net
Received date: April 03, 2016; Accepted date: April 27, 2016; Published date: April 29, 2016
Citation: Santiago-Burruchaga M, Catediano-Sainz E, Jorge RZ, Cordero CV. Bronchoscopic Abnormalities in Pediatric Chronic Cough: A Case-Control Study. J Pediatric Infect Dis. 2016, 1:10. doi: 10.21767/2573-0282.100010
Copyright: © 2016 Santiago-Burruchaga M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Lower airway abnormalities, inflammation and bacterial infection are frequently described in children with chronic cough but its frequency in asymptomatic children is unknown. We report bronchoscopic abnormalities observed in children with chronic cough and in children undergoing fiberoptic bronchoscopy for causes other than chronic cough. Methods and findings: Retrospective case-control cohort study. The clinical records of children who had fiberoptic bronchoscopy for a history of chronic cough between 2008 and 2014 and of control children who required fiberoptic bronchoscopy for other causes were reviewed. Bronchoscopic findings and bronchoalveolar lavage results were assessed. Cases were 50 [27 female, median age 5.5 (0.5-12) years]. Their cough was wet in 37 (74%). Controls were 29 [12 female, median age 5.6 (0.5-14) years]. Airway malacia was observed in 25 cases (50%) vs 4 controls (14%) (p=0.001) and profuse secretions in 22 (44%) vs 6 (21%) (p = 0.03). When bronchoalveolar lavage was performed relevant organisms were isolated in 18 (45%) cases vs 4 (18%) controls (p=0.03). H influenzae (9), M catarrhalis (4) and S pneumonia (3) were seen only in cases. Polymerase chain reaction to viruses was positive in 13 (33%) cases vs 4 (14%) controls (p=0.04). Similar viruses were found in both: Rhinovirus and Adenovirus. Conclusions: Airway abnormalities were observed in seven out of ten cases the commonest one being airway malacia. It was associated with a higher frequency of positive cultures mostly for non typable H. influenzae and M. catarrhalis which were not isolated in controls. Similar viruses (Rhinovirus and Adenovirus) were found in both groups, although isolation was more frequent in cases.
Keywords
Respiratory system abnormalities; tracheobronchomalacia; bronchoalveolar lavage; respiratory tracts infections
Introduction
Chronic cough (CC) is usually defined as one which going on for longer than four weeks, 1 as cough associated with uncomplicated acute respiratory infections often resolves within this time limit. The assessment of children with CC should be based on current Paediatric Clinical Guidelines [1,2]. The “watch, wait and review” first step in the 2006 ACCP guidelines 1 is usually appropriate. Clues of underlying conditions should be investigated, namely: a history of specific cough, abnormalities at physical examination, and/or alterations in simple ancillary tests [3,4]. In some cases the presence of serious underlying disorders can be suspected and once the patient is referred to a respiratory specialist a fiberoptic bronchoscopy (FB) can be considered [5].
Protracted bacterial bronchitis (PBB) and non-specific CC make up the bulk of diagnosis in children with CC [6]. Nonspecific self-resolving CC is largely an exclusion diagnosis, it’s likely cause being post-infectious in most instances. Coughvariant asthma, that is cough as the sole manifestation of bronchial asthma, and gastroesophageal reflux, which are common causes in adults, are not so to the same extent in children [6].
PBB is the most common cause of specific CC in children. Recent Clinical Guidelines, [7] and evidence systematic reviews [8] no longer require a positive bronchoalveolar lavage (BAL) result to diagnose PBB, which can be made in the face of cough resolution following an antibiotic course and no evidence of another origin of CC being sufficient. Its natural history and long/term outcome is largely unknown. In some cases, as proposed by the "vicious circle hypothesis", defective mucocilliary clearance combined with chronic neutrophilsdominated inflammation may lead to progressive airways damage and bronchiectasis [9]. A high prevalence of medium and lower airways malacia has been reported in such cases [10,11]. Airways malacia can put patients at risk of suffering protracted bronchitis and ultimately developing bronchiectasis by interfering with pivotal pulmonary defense mechanisms like mucus clearance and effective coughing [12]. The scarcity of reports of bronchoscopic findings in control healthy children because of the ethical difficulties for performing invasive procedures in this population prevents comparing their incidence of malacia with that found in children with CC.
The aim of our study was to describe bronchoscopic abnormalities observed in children with chronic cough and in children undergoing fiberoptic bronchoscopy for causes other than chronic cough.
Materials and Methods
Both clinical records and FB findings in children who had CC without any known underlying condition referred for assessment to the Paediatric Pulmonology Outpatient Clinics at a Tertiary Teaching Hospital in whom FB was indicated since 2008 through 2014 were reviewed. The management of the patients was based on current Pediatrics Guidelines on CC [1,2]. Children with known concomitant chronic lung, or complex congenital heart disease, or relevant neurologic or developmental disorders were excluded. A control group of children without a history of CC who had FB for other causes over the same time span and fulfilled the same exclusion criteria were also studied. This study was approved for its publication by the Clinical Research and Ethics Committee at Cruces Hospital University.
Study process
Patient’s age, sex, clinical history, cough characteristics and duration, associated diagnosis, ancillary tests results, and FB and BAL findings were all recorded. Additionally, the total number of all performed FB procedures since 2008 through 2014 were collected. FB (Olympus Video bronchoscope) was performed on a scheduled basis, on spontaneous breathing under analgesia, sedation and local anesthesia at the Intensive Care Unit. The bronchoscopic technique used was the same in both groups. Images were video recorded and reviewed later on. BALs were performed following the European Respiratory Society Guidelines [13]. Samples were shipped to the Laboratory for Gram staining, and counting of any bacteria colony forming units (CFU). CFU ≥ 104 cc was considered as reflecting endobronchial infection. Any growth of normal oropharyngeal organisms was disregarded. Respiratory viruses were investigated by polymerase chain reaction (PCR) and BAL culture.
Definitions
CC: Cough lasting for longer than 4 weeks.
CC characteristics - as described by parents and observed at the office: wet (“shifting” or producing secretions), dry (no secretions-shifting sound), “brassy" (metallic sound typical of laryngotracheal cough).
PBB: (1) the presence of isolated chronic wet/moist cough, (2) resolution of cough with antimicrobial treatment, and (3) absence of pointers suggestive of an alternative specific cause of cough.
Airways malacia: Over 70% (severe if ≥ 90%) airway lumen dynamic expiratory collapse either on spontaneous breathing or during coughing while no aspiration was being applied.
Bronchial stenosis: fixed bronchial lumen narrowing as compared with neighbouring bronchi.
Bronchial inflammation signs (bronchitis): Abnormal amount of secretions.
Statistical analyses
Children were split into two groups: with or without CC as defined above. PSS for Windows, version 11.0 (SPSS Inc., Chicago, IL, USA) was used. Numeric variables were described as mean and median and categories as frequencies and percentages. Chi square, Student's t test and Mann Whitney tests, when appropriate were used to assess differences between groups; p values <0.05 were considered significant. Sensitivity and specificity of chronic wet cough for the finding of bronchial secretions and of chronic brassy cough for malacia were calculated.
Results
Fifty (27 female) children with CC, median age 5.5 (0.5-12) years, had FB on a scheduled basis since 2007 through 2014 making up 8.4% of all performed FB. Their cough was wet in 37 (74%), brassy in 7 (14%), and dry in 6 (12%). It had been going on for 16 months (1-120), exceeding 6 months in 44 (88%). Thirteen (26%) had had previously recurrent pneumoniae. Ten (20%) had abnormal chest X-ray and/or chest CT scan: 3 (6%) subsegmental atelectasis, 2 (4%) cylindrical bronchiectasis, 2(4 %) segmental bronchial stenosis and one each: lung hyperinflation, mosaic pattern of hyperlucency, and aberrant subclavia artery. Four out of eighteen (22%) old enough to collaborate at spirometry, had an abnormal ventilator pattern: restrictive, mixed or flattening of forced flow-volume loops.
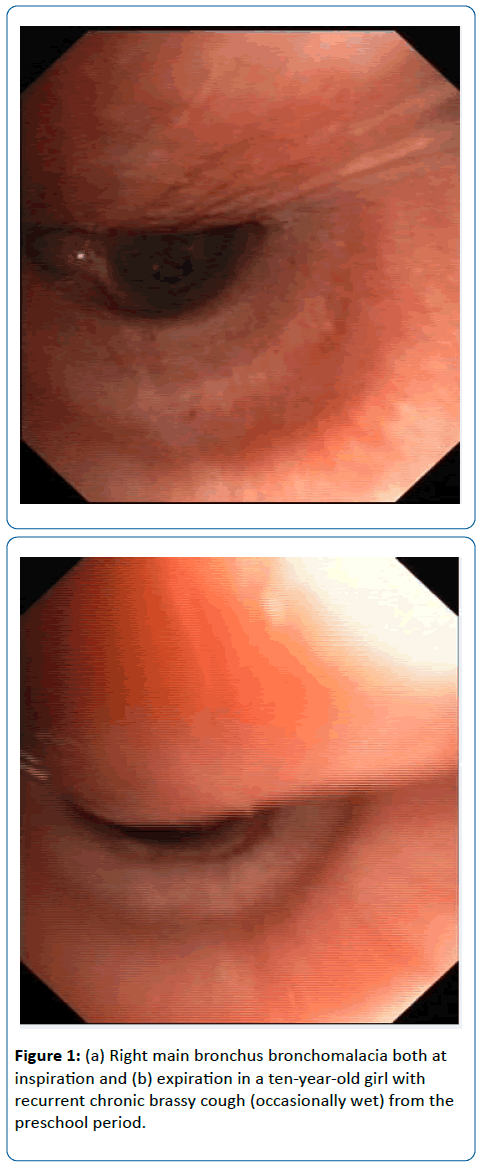
Thirty (60%) had structural airways abnormalities (SAA) at FB: lower airways malacia 25 (50%) (Figures 1a and 1b) [9 (36%) tracheomalacia, 9 (36%) tracheobronchomalacia, and 6 (24%) bronchomalacia], segmental bronchial stenosis 5 (10%) and miscellaneous 5 (10%): inflammatory bronchial granulomas 2 (4%), tracheal stenosis and tracheal bronchus, tracheobronchomegalia, and lingual tonsils hypertrophy. Malacia was severe in 13 (52%). BAL was performed in 40 (80%). Twenty-one relevant organisms were identified in 18 children (45%): non-typable Haemophilus influenzae (11), Moraxella catarrhalis (4), Streptococcus pneumoniae (3), nonmucoid Pseudomonas aeruginosa (2) and Staphylococcus aureus. PCR to one or several respiratory viruses was positive in 13 (32.5%): Rhinovirus (7), Adenovirus (5), RSV (4), Parainfluenza (2), and one each: Bocavirus, Metapneumovirus, Coronavirus, and Influenza virus. Seven (54%) of those with a respiratory viruses positive PCR had concomitant bacterial infection. Children with positive BAL bacterial culture had more frequently SAA (50% vs 9%, p=0.000) [lower airways malacia 59% vs 28%, p=0.02] and evidence of increased bronchial secretions at FB (76% vs 24%, p= 0.00). A history of chronic wet cough had 83% sensitivity but only 37% specificity for the finding of increased bronchial secretions at FB, and 72% sensitivity and 33% specificity for a positive BAL bacterial culture. A history of chronic brassy cough had only 25% sensitivity but 90% specificity for lower airways malacia. Two out of six (33%) with a history of dry cough had SAA (one each bronchomalacia and lingual tonsils hypertrophy).
Controls were 29 children (12 female) [aged 5.6 year (0.5-14)]. Twenty-one (72%) had FB in the course of a hospital admission and eight (28%) were scheduled from outpatient clinics. Twenty-one (72%) had pneumonia poorly responsive to therapy, five (17%) not confirmed suspected endobronchial foreign body inhalation and one each hemoptysis, massive atelectasis and pulmonary hemorrhage. Sixteen (55%) had underlying oncological conditions. Six (20%) had lower airways abnormalities at FB: malacia 4 (14%) [tracheobronchomalacia and bronchomalacia (3)], bronchial stenosis 3 (10%) (1had associated bronchomalacia). Six (21%) had evidence of bronchitis (3 of them with associated bronchomalacia). BAL was performed in 22 (76%) and three potentially relevant organisms were grown in 4 (18%): Pneumocystiis jirovecii (2), Streptococcus mitis, and Streptococcus oralis. PCR to one or several respiratory viruses was positive in 4 (18%): Rhinovirus (3) y Adenovirus. Two of those with respiratory viruses positive PCR had concomitant bacterial infection. Differences between groups are shown on Table 1.
| Cases | Controls | P | |
|---|---|---|---|
| Total number (femela) | 50 (27) | 29 (12) | - |
| Median age (years) | 5.5 (0.5-12) | 5.6 (0.5-14) | ns |
| Airways abnormalities | 30 (60) | 6 (20) | 0.001 |
| Lower airway malacia | 25 (50) | 4 (14) | 0.001 |
| Segmental bronchial stenosis | 5 (10) | 3 (10) | ns |
| Other abnormalities | 5 (10) | - | - |
| Brochitis | 22 (44) | 6 (21) | 0.03 |
| Bacterial isolation | 18 (45) | 4 (18) | 0.03 |
| Viruses isolation (PCR) | 13 (32) | 4 (14) | 0.04 |
Table 1: Patients and bronchoscopic findings.
Discussion
Unlike adults with CC in whom FB has not been shown to be useful [14]. FB should be considered in selected pediatric patients once non-invasive approaches have failed to establish diagnosis, in order to examine airways patency and structure, and obtain biological samples for microbiology and inflammatory pattern investigations. 5 Its precise indications can vary among centres depending on the individual diagnosis algorithms used. As a whole, our cases accounted for 8.4% of all FB performed during the same time span, a figure slightly lower than that reported by De Blic et al. (11.6%) in a large pediatric cohort [15].
Nine out of every ten had had either a wet or brassy cough for longer than six months, one out every three had either imaging or pulmonary function tests abnormalities, and one out of every four had had recurrent pneumonia. Dry CC is often non-specific and of post-infectious origin, commonly resolving spontaneously and it is seldom an indication for FB [16]. Still cases with chronic dry cough amounted to 12% of all having FB and in two associated SAA were observed.
Conversely reported chronic wet/productive cough is a good marker for endobronchial neutrophils-dominated inflammation and infection [17,18]. It was reported in seven out of ten of our cases, and showed high sensitivity (83%) for inflammatory signs at FB and, to a lesser extent (72%), for endobronchial bacterial infection.
BAL was performed in eight out of every ten, and relevant pathogenic organisms were recovered in 45% of cases, its frequency being far higher than in controls. The three species more frequently causing chronic respiratory infections comprised eighty percent of the isolates. Non-capsulate Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pneumonia all are common nasopharynx colonizers. They were not isolated in controls. Bacterial growth in BAL samples correlated with evidence of endobronchial inflammation as previously reported [18,19]. Whether recurrent protracted episodes of endobronchial infection can give rise to chronic suppurative pulmonary disease and ultimately bronchiectasis remains to be determined [19]. Only two (4.7%) of our patients had evidence of bronchiectasis. Still it should be born in mind that progresssion to bronchiectasis may take years or even decades.
Respiratory viruses were identified in one out of three cases including half of those with a positive bacterial culture. Although isolation was more frequent in this group than in the controls, similar viruses found in both: Rhinovirus and Adenovirus. Respiratory viruses in addition to their pathogenic effect can enhance bacterial adhesion to bronchial epithelial, and interfere with bacterial killing [20] Rhinoviruses was the commonest species as reported by others. Rhinovirus replication and persistence has been reported in some chronic diseases, as dilated cardiomiopathy, and correlated with poor outcome [21] Adenovirus followed rhinovirus in frequency. Its association with PBB and its possible role in its pathogenesis has been previously reported [22].
Lower airways malacia was the commonest abnormality found and its frequency was far higher than that observed in our control group. As there are no precise objective diagnostic criteria for these conditions to be used in everyday clinical practice, we have relied on the comparatively subjective assessment of a moderate or severe (over 70%) collapse of the airways lumen, in order to disregard borderline cases. Our observed prevalence is close to that reported by Kompare et al in children with PBB. 12 Eight out ten of those with lower airways malacia had tracheal or tracheobronchial involvement. Wurzel et al 23 found a high prevalence (68%) of lower airways malacia in a prospective study in children with PBB, and in a control group (53%) of children with chronic respiratory symptoms and high prevalence of endobronchial infection. Our control group was composed by children without a history of CC but who required FB by acute respiratory symptoms. Their prevalence of lower airways was significantly lower with just one having tracheomalacia.
It is remarkable that one out every ten observed SAA were other than lower airways malacia and were not observed in controls. While other tracheal abnormalities [stenosis and tracheal bronchus, and trachebronchomegaly and lingual tonsils hypertrophy impinging on the epiglottis is rarely cause of chronic cough in children, as a whole their prevalence was not negligible, and they are probably underdiagnosed.
Children with SAA had more frequently inflammatory findings at FB and endobronchial infection, and a sizeable subgroup had a history of recurrent pneumonia, both suggesting likely long lasting airways infection-inflammation. Bronchial granulomas and segmental bronchial stenosis are often secondary to protracted inflammation. On the contrary airways malacia, while sometimes especially when segmental bronchi are involved might be secondary to chronic inflammation [23] it is mostly thought to be a primary cause of chronic airways inflammation, ineffective coughing and disturbed mucus clearance contributing to perpetuating chronic endobronchial infection-inflammation [12]
Our study, being retrospective, has major limitations. While for the management of the patients we followed standard Paediatric Clinical Guidelines [1,2] we lacked a written protocol. A lot of patients, mainly in control group, were on treatment or prophylactic antibiotics prior to FB so we cannot estimate their potential impact on BAL culture and the presence of inflammatory signs.
We conclude that FB in selected children with CC had a high yield as structural abnormalities were observed in seven out of ten the commonest one being lower airways malacia, mostly tracheomalacia. Its incidence was in keeping with that previously reported and far higher than that observed in the control group. It was associated with endobronchial infection with H. influenzae, M.catarrhalis or S. pneumonia. These organisms were not isolated in controls. Similar viruses (Rhinovirus and Adenovirus) were found in both groups although isolation was more frequent in cases. Important research questions remain to be answered about biological susceptibility factors, including the role of airway malacia and the pathobiologic mechanisms of virus-induced episodes.
Statement of author´s contributions
All the authors were involved in the study design, date collection and writing of the manuscript.
References
- Chang AB, Glomb WB (2006) Guidelines for Evaluating Chronic Cough in Pediatrics. ACCP Evidence-Based Clinical Practice Guidelines. Chest 129: 260S-283S.
- Shields MD, Bush A, Everard ML, McKenzie S, Primhak (2008) on behalf of the British Thoracic Society Cough Guideline Group. Recommendations for the assessment and management of cough in children. Thorax 63: 1-15.
- Chang AB, Robertson CF, Van Asperen PP, Glasgow NJ, Masters IB, et al. (2013) A cough Algorithm for Chronic Cough in Children: A Multicenter, Randomized Controlled Study. Pediatrics 131: 1576-1583.
- Chang AB, Van Asperen PP, Glasgow N, Robertson CF, Mellis CM, et al. (2015) Children with Chronic Cough. When is watchful waiting appropriate? Development of likelihood ratios for assessing children with chronic cough. Chest 147: 745-753.
- Masters IB, Chang AB (2010) Chronic cough. In: Priftis KN, Anthracopoulos MB, Eber E, Koumbourlis AC, Wood RE, eds. Paediatric bronchoscopy. ProgRespir Res. Basel, Karger 38: 182-190.
- Yilmaz O, Bakirtas A, ErtoyKaragol HI, Topal E, Turktas I (2014) Children with chronic non-specific isolated cough. Chest 145: 1279-1285.
- Chang AB, Landau LI, Van Asperen PP, Glasgow NJ, Robertson CF, et al. (2006) Thoracic Society of Australia and New Zealand Cough in children: definitions and clinical evaluation. Med J Aust. 184 : 398-403.
- Marchant JM, Morris P, Gaffney J, Chang AB (2005) Antibiotics for prolonged moist cough in children. Cochrane DatebaseSyst Rev 19: CD004822
- Craven V, Everard ML (2013) Protracted bacterial bronchitis: reinventing an old disease. Arch Dis Child 98: 72-76.
- MarchantJm, Masters IB, Taylor SM (2006) Evaluation and outcome of young children with chronic cough. Chest pp 129:1132.
- Kompare M, Weinberger M (2012) Protracted bacterial bronchitis in young children: Association with airway malacia. J Pediatr 160: 88-92.
- Donnelly D, Critchlow A, Everard ML (2007) Outcomes in children treated for persistent bacterial bronchitis. Thorax 62: 80-84.
- De Blic J, Midulla F, Barbato A, Clement A, Dab I, et al. (2000) Bronchoalveolar lavage in children. ERS Task Force on bronchoalveolar lavage in children. European Respiratory Society. EurRespir J 15: 217-231.
- Barnes TW, Afessa B, Swanson KL, Lim KG (2004) The clinical utility of flexible bronchoscopy in the evaluation of chronic cough. Chest 126: 268-272
- De Blic J, Marchac V, Scheinmann P (2002) Complications of flexible bronchoscopy in children prospective study of 1328 procedures. EurRespir J 20: 1271-1276.
- Shields MD, Doherty GM (2013) Chronic cough in children. Paediatric Respiratory Reviews 14: 100-106.
- Wurzel DF, Marchant JM, Clark JE, Masters IB, Yerkovich ST, et al. (2014) Wet cough in children: Infective and inflammatory characteristics in Broncho-Alveolar lavage fluid. Pediatric Pulmonology 49: 561-568.
- Zgherea D, Pagala S, Mendiratta M, Michael G. Marcus, Steven P. Shelov, et al. (2012) Bronchoscopic findings in children with chronic wet cough. Pediatrics 129: 364-369.
- Chang AB, Yerkovich ST, Gibson PG, Anderson-James S, Petsky HL, et al. (2012) Pulmonary innate immunity in children with protracted bacterial bronchitis. J Pediatr 161: 621-625.
- Grimwood K (2011) Airway microbiology and host defences in paediatric non CF bronchiectasis. Paediatric Respiratory Reviews 12: 111-118.
- Bugin S, Lunardi F, Bertuola F, Snijders D, Bottecchia L, Perissinotto E, et al. (2013) Pediatric chronic lower respiratory disorders: Microbiological and inmunological phenotype. Pediatric Pulmonology 48: 78-788.
- Wurzel DF, Marchant JM, Yerkovich ST, Upham JW, Mackay IM, et al. (2014) Prospective characterization of protracted bacterial bronchitis in children. Chest 145: 1271-1278.
- Chang AB, Boyce NC, Masters IB, Torzillo PJ, Masel JP (2002) Bronchoscopic findings in children with non-cystic fibrosis chronic suppurative lung disease. Thorax 57: 935-938.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences